

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087536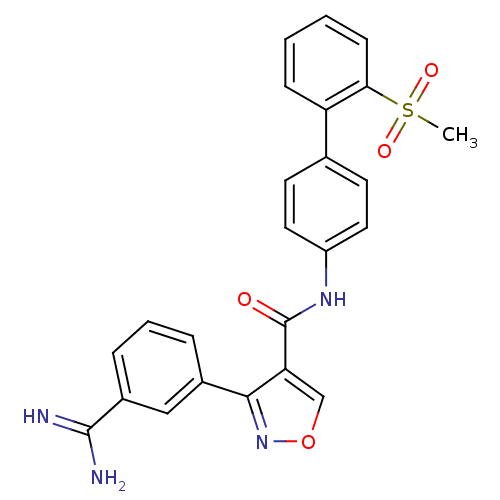 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087533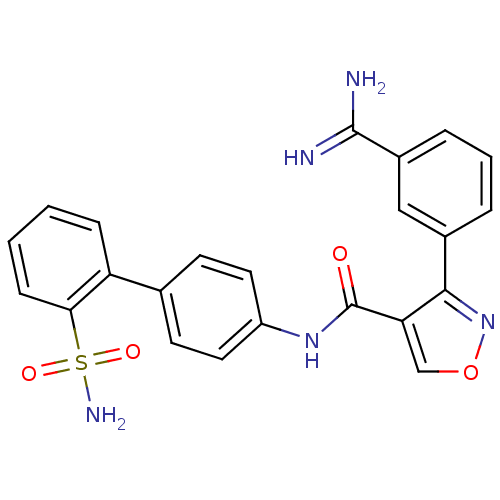 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50087533 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against rabbit Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087540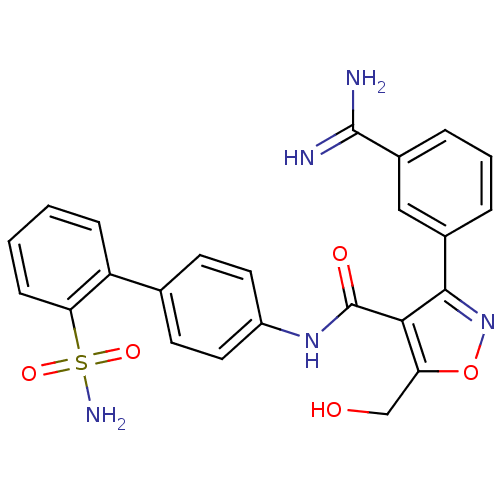 (3-(3-Carbamimidoyl-phenyl)-5-hydroxymethyl-isoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087538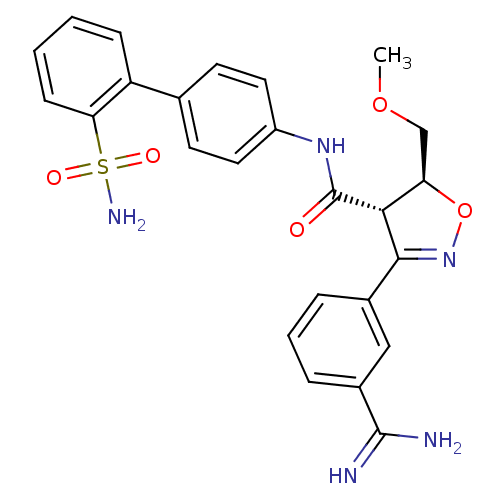 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087532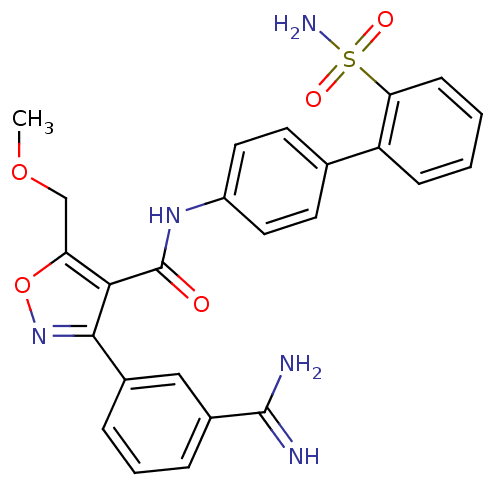 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50087538 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against rabbit Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087535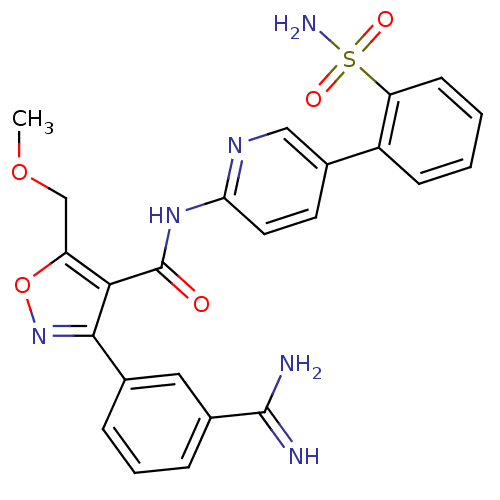 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Oryctolagus cuniculus) | BDBM50079252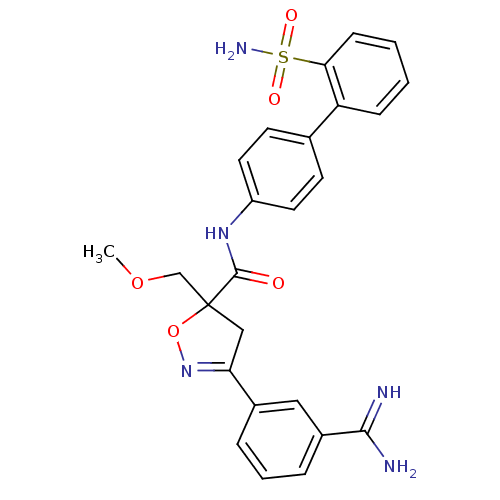 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-4,5-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was tested against rabbit Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079236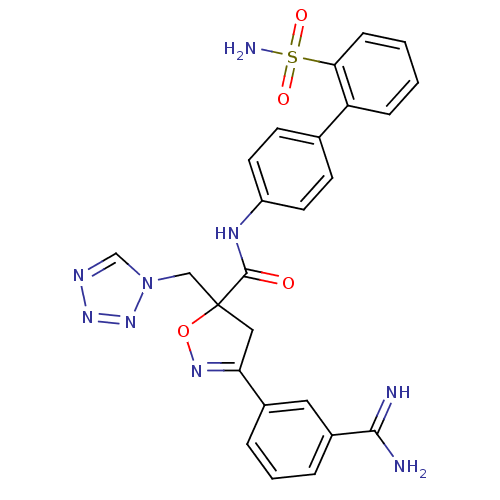 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087539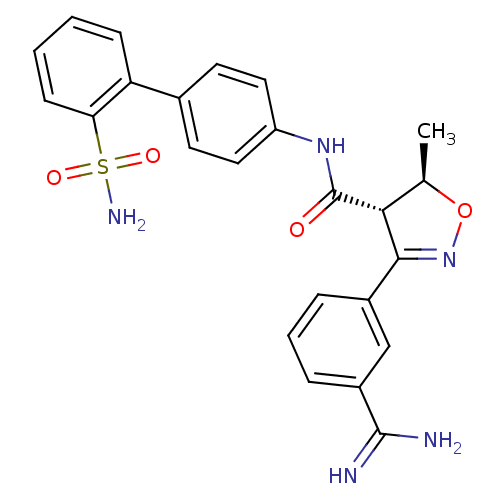 ((4S,5R)-3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087537 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087528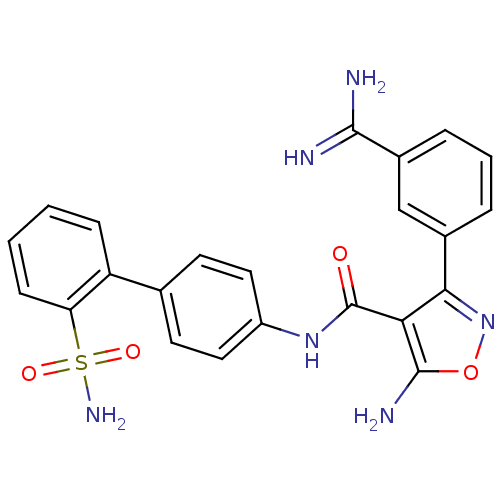 (5-Amino-3-(3-carbamimidoyl-phenyl)-isoxazole-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079252 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50087534 (3-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-isoxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50079247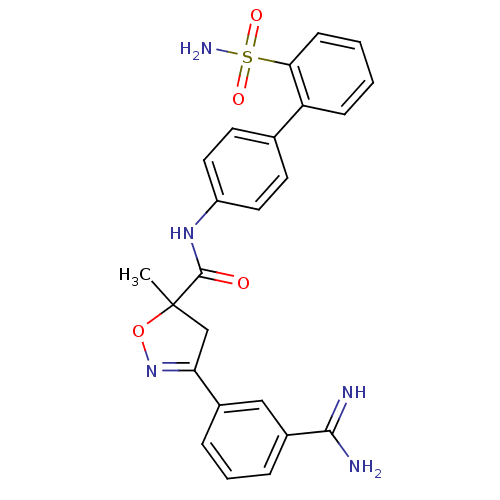 (3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-dihydro-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of human Coagulation factor X | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087533 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087536 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087540 (3-(3-Carbamimidoyl-phenyl)-5-hydroxymethyl-isoxazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50079252 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-4,5-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087532 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50079236 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087539 ((4S,5R)-3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087528 (5-Amino-3-(3-carbamimidoyl-phenyl)-isoxazole-4-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50079247 (3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-dihydro-is...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087537 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087538 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087535 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50087534 (3-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-isoxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087536 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079236 (3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-ylmethyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087540 (3-(3-Carbamimidoyl-phenyl)-5-hydroxymethyl-isoxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087533 (3-(3-Carbamimidoyl-phenyl)-isoxazole-4-carboxylic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079252 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-4,5-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087532 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087528 (5-Amino-3-(3-carbamimidoyl-phenyl)-isoxazole-4-car...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087539 ((4S,5R)-3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087538 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087537 ((4S,5S)-3-(3-Carbamimidoyl-phenyl)-5-tetrazol-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50079247 (3-(3-Carbamimidoyl-phenyl)-5-methyl-4,5-dihydro-is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087534 (3-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-isoxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50087535 (3-(3-Carbamimidoyl-phenyl)-5-methoxymethyl-isoxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 10: 685-9 (2000) BindingDB Entry DOI: 10.7270/Q21V5D63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||