

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50088653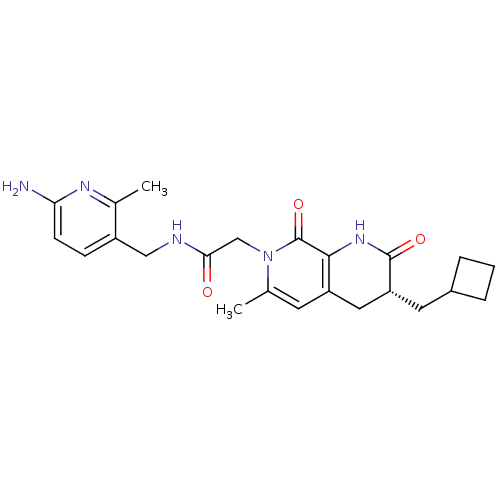 (CHEMBL274152 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088660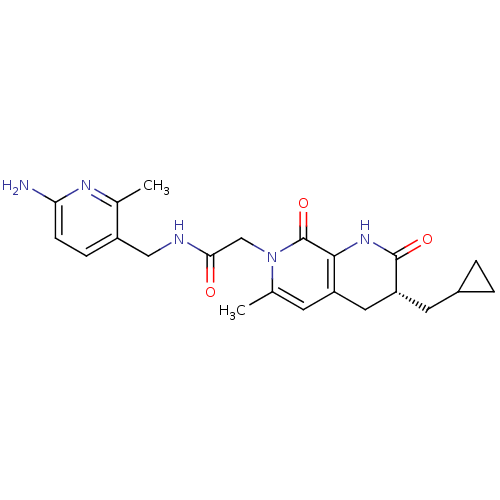 (CHEMBL10785 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657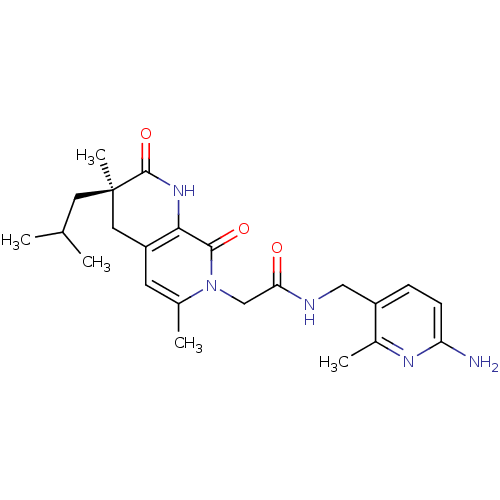 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088659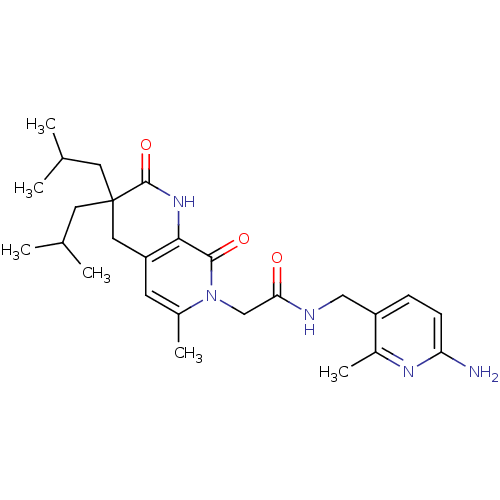 (CHEMBL10346 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088658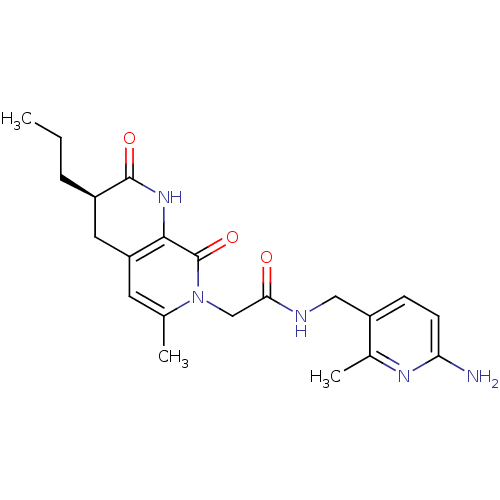 (CHEMBL10800 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088655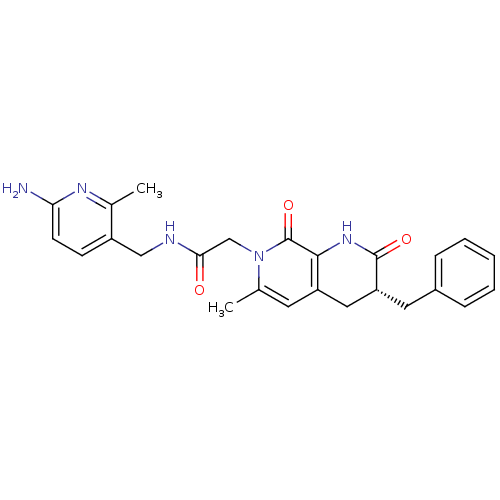 (CHEMBL10545 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088652 (CHEMBL275520 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088656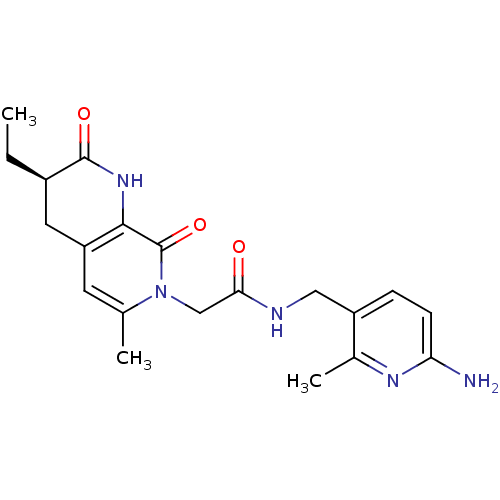 (CHEMBL10657 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088661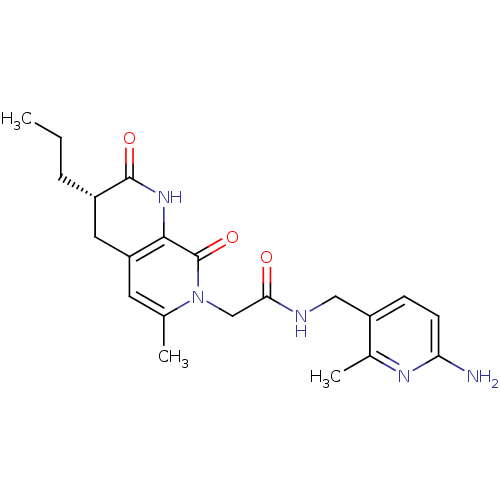 (CHEMBL275110 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088654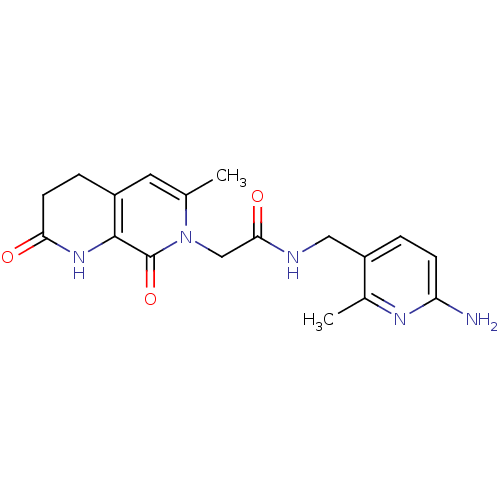 (CHEMBL273861 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088653 (CHEMBL274152 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088658 (CHEMBL10800 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088652 (CHEMBL275520 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088660 (CHEMBL10785 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088659 (CHEMBL10346 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088656 (CHEMBL10657 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088655 (CHEMBL10545 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088661 (CHEMBL275110 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088654 (CHEMBL273861 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||