

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317868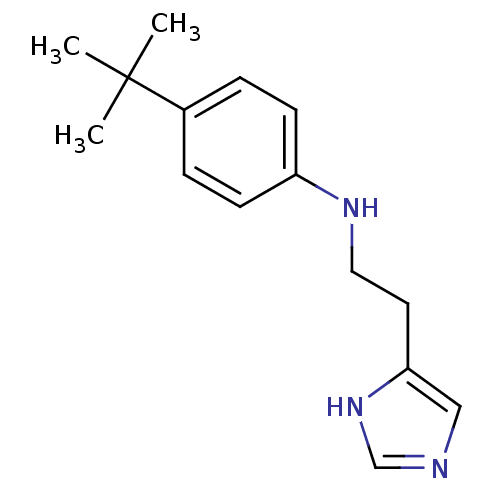 (4-(2-(4-tert-Butylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317864 (4-(2-(4-Trifluoromethylphenylamino)ethyl)-1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant histamine H4 receptor expressed in CHO cells | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317867 (4-(2-(3-tert-Butylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317866 (4-(2-(4-Cyclohexylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317869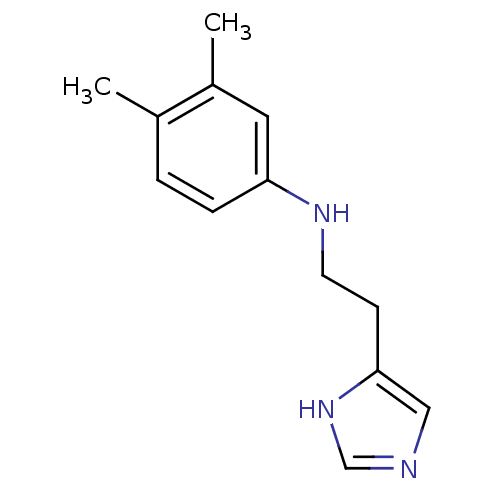 (4-(2-(3,4-Dimethylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317870 (4-(2-(4-Methylphenylamino)ethyl)-1H-imidazole | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317863 (4-(2-(4-Methoxyphenylamino)ethyl)-1H-imidazole | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317871 (4-(2-(Phenylamino)ethyl)-1H-imidazole | CHEMBL1096...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of beta-1 adrenergic receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of dopamine D3 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of beta2 adrenergic receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of muscarinic M1 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of muscarinic M3 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of 5HT1A receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of dopamine D2 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of 5HT3 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of 5HT2A receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of dopamine D1 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of histamine H2 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of histamine H1 receptor | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317872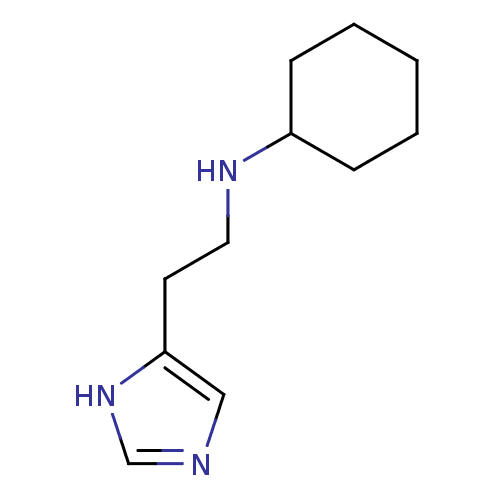 (4-(2-(Cyclohexylamino)ethyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in insect cell microsome after 30 mins by fluorescence assay | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in insect cell microsome after 30 mins by fluorescence assay | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in insect cell microsome after 15 mins by fluorescence assay | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in insect cell microsome after 30 mins by fluorescence assay | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317870 (4-(2-(4-Methylphenylamino)ethyl)-1H-imidazole | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317869 (4-(2-(3,4-Dimethylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317868 (4-(2-(4-tert-Butylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317867 (4-(2-(3-tert-Butylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317864 (4-(2-(4-Trifluoromethylphenylamino)ethyl)-1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317872 (4-(2-(Cyclohexylamino)ethyl)-1H-imidazole | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317863 (4-(2-(4-Methoxyphenylamino)ethyl)-1H-imidazole | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317871 (4-(2-(Phenylamino)ethyl)-1H-imidazole | CHEMBL1096...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||