

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103087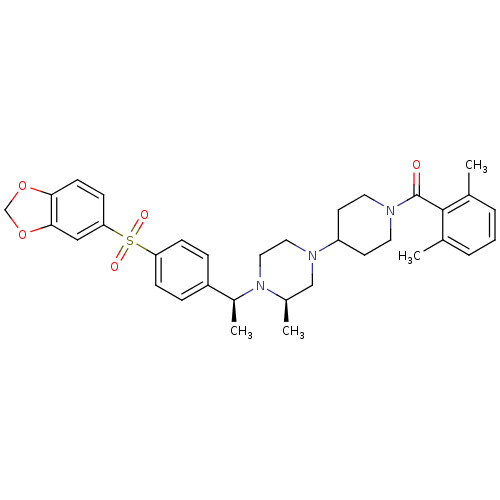 (CHEMBL305026 | [4-((R)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103078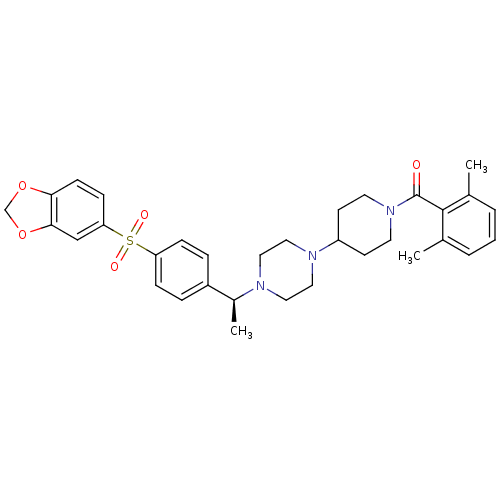 (CHEMBL67563 | [4-(4-{(S)-1-[4-(Benzo[1,3]dioxole-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103083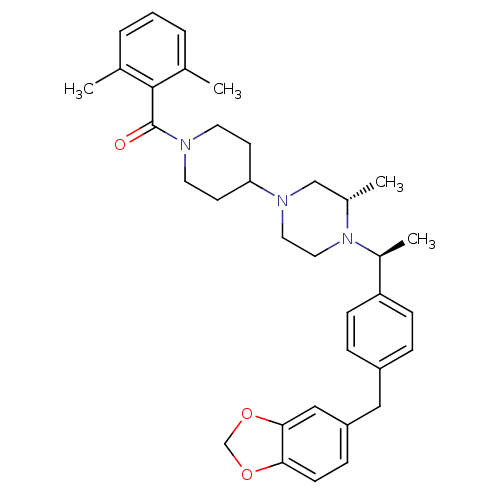 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103088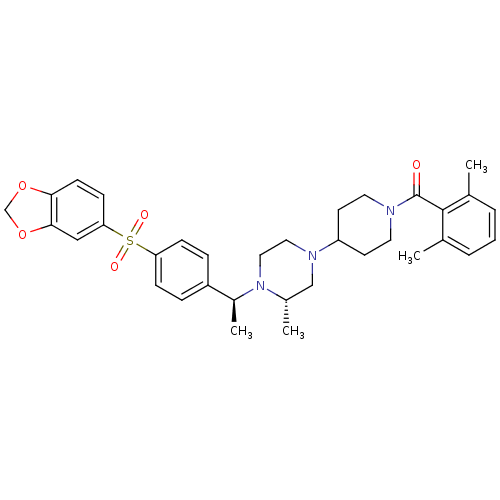 (CHEMBL70010 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]dioxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103091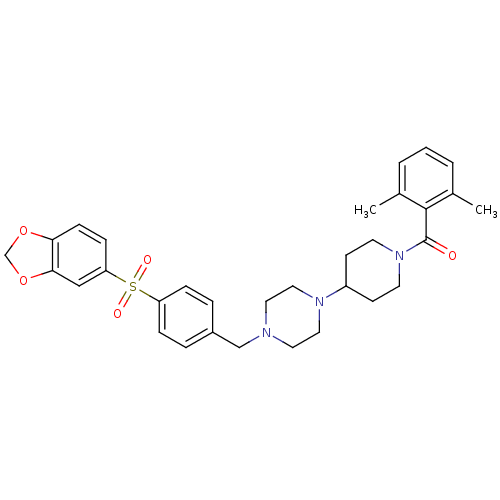 ((4-{4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl]-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103080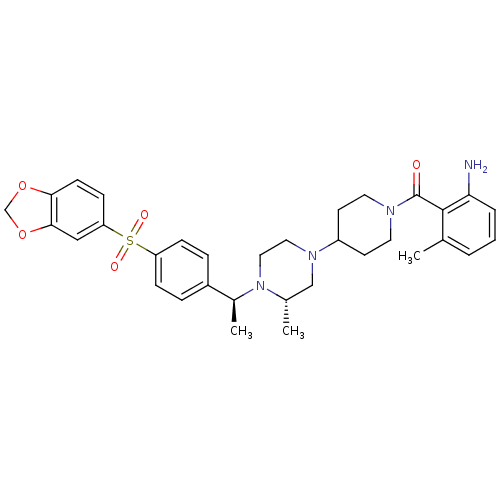 ((2-Amino-6-methyl-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of the compound to inhibit [125I]-labeled RANTES binding to C-C chemokine receptor type 5 expressed in membrane preparation of CHO cells | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103085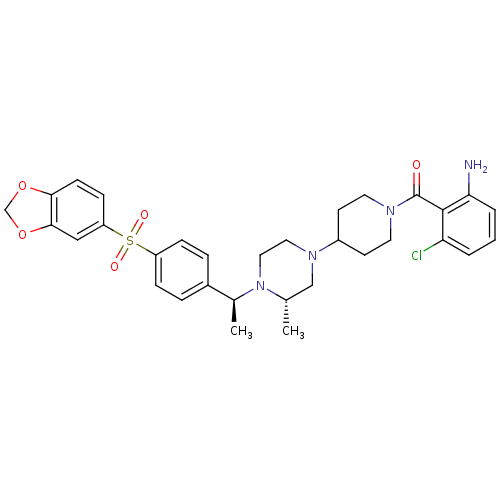 ((2-Amino-6-chloro-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103090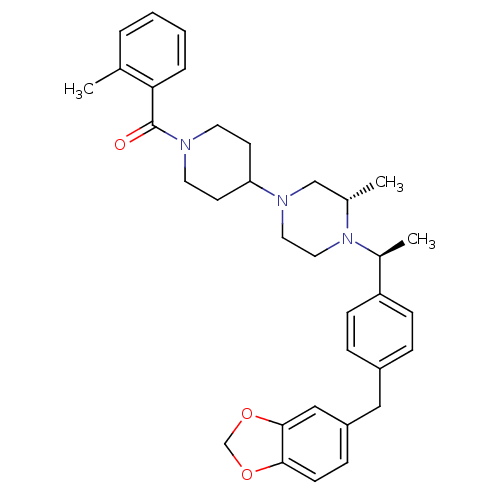 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103084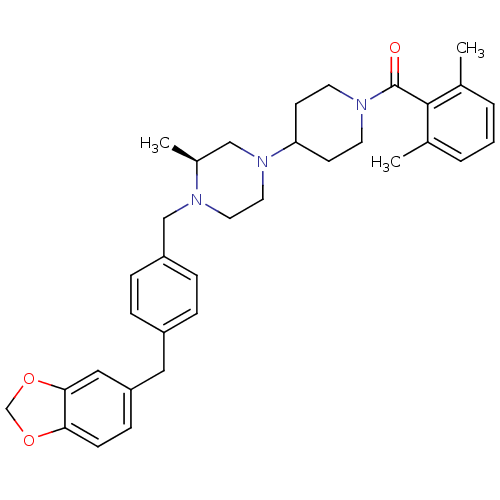 (CHEMBL66623 | {4-[(S)-4-(4-Benzo[1,3]dioxol-5-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103089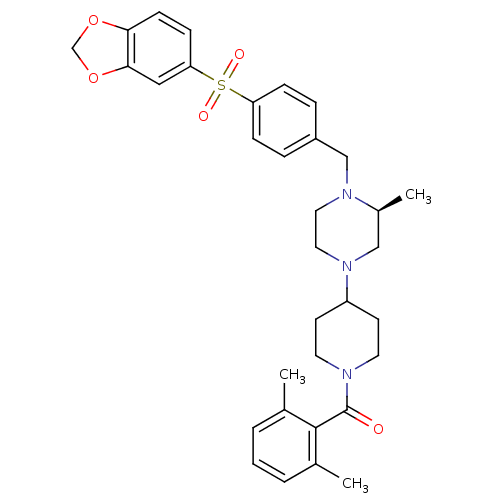 ((4-{(S)-4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103084 (CHEMBL66623 | {4-[(S)-4-(4-Benzo[1,3]dioxol-5-ylme...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103086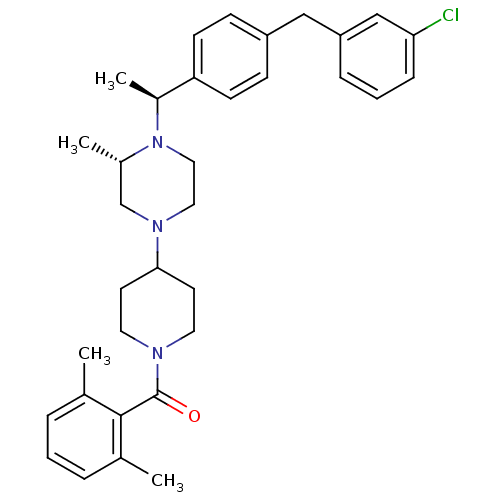 (CHEMBL413972 | [4-((S)-4-{(S)-1-[4-(3-Chloro-benzy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103079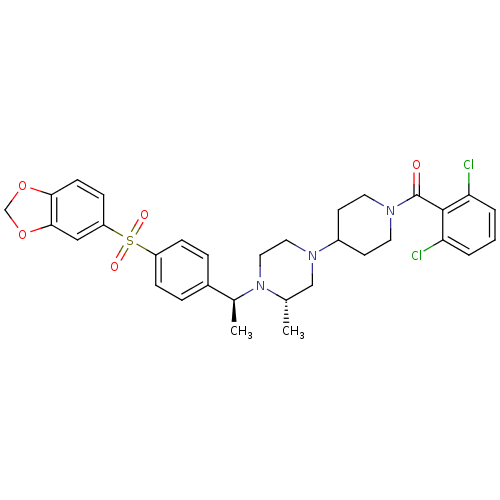 (CHEMBL303503 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103089 ((4-{(S)-4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103085 ((2-Amino-6-chloro-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103080 ((2-Amino-6-methyl-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103082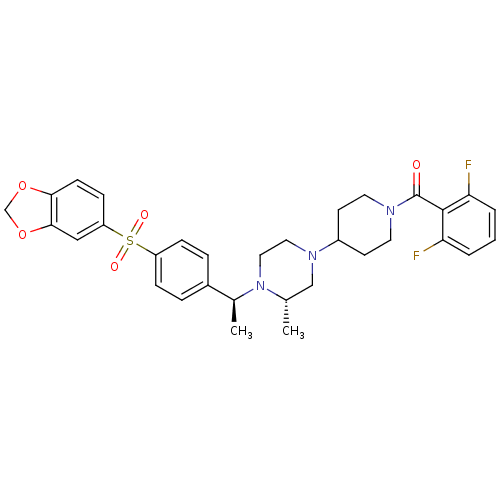 (CHEMBL67281 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]dioxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103079 (CHEMBL303503 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103082 (CHEMBL67281 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 352 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103081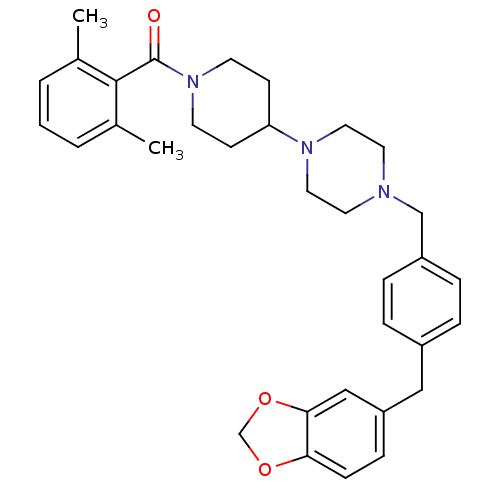 (CHEMBL63757 | {4-[4-(4-Benzo[1,3]dioxol-5-ylmethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 352 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103078 (CHEMBL67563 | [4-(4-{(S)-1-[4-(Benzo[1,3]dioxole-5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 442 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103088 (CHEMBL70010 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 485 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103081 (CHEMBL63757 | {4-[4-(4-Benzo[1,3]dioxol-5-ylmethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103083 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103091 ((4-{4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl]-pi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103086 (CHEMBL413972 | [4-((S)-4-{(S)-1-[4-(3-Chloro-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103090 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of the compound to inhibit [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103087 (CHEMBL305026 | [4-((R)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of the compound to inhibit [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||