 Found 36 hits Enz. Inhib. hit(s) with all data for entry = 50011520
Found 36 hits Enz. Inhib. hit(s) with all data for entry = 50011520 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107512
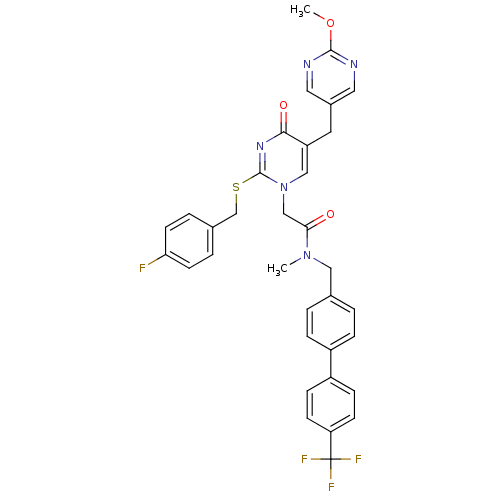
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(cc3)C(F)(F)F)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H29F4N5O3S/c1-42(18-22-3-7-25(8-4-22)26-9-11-28(12-10-26)34(36,37)38)30(44)20-43-19-27(15-24-16-39-32(46-2)40-17-24)31(45)41-33(43)47-21-23-5-13-29(35)14-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107505
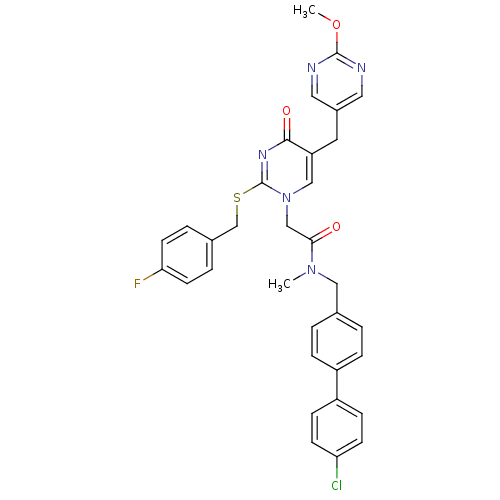
(CHEMBL79555 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2-...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H29ClFN5O3S/c1-39(18-22-3-7-25(8-4-22)26-9-11-28(34)12-10-26)30(41)20-40-19-27(15-24-16-36-32(43-2)37-17-24)31(42)38-33(40)44-21-23-5-13-29(35)14-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107480
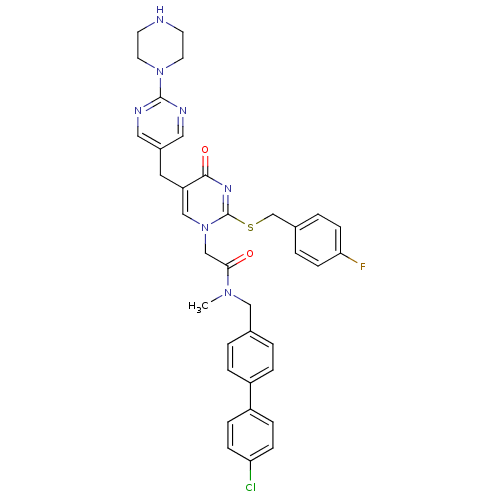
(CHEMBL348243 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(nc2)N2CCNCC2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H35ClFN7O2S/c1-43(21-25-2-6-28(7-3-25)29-8-10-31(37)11-9-29)33(46)23-45-22-30(18-27-19-40-35(41-20-27)44-16-14-39-15-17-44)34(47)42-36(45)48-24-26-4-12-32(38)13-5-26/h2-13,19-20,22,39H,14-18,21,23-24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107507
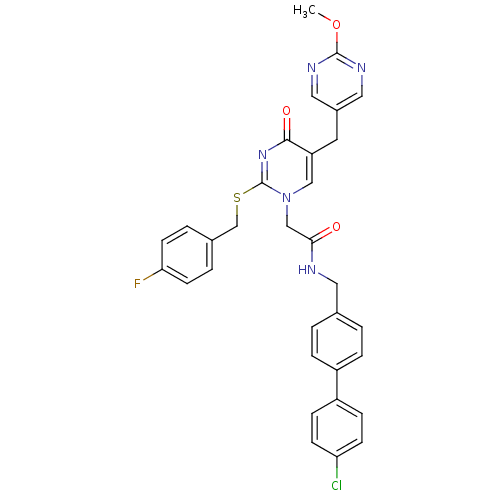
(CHEMBL358483 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES COc1ncc(Cc2cn(CC(=O)NCc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C32H27ClFN5O3S/c1-42-31-36-16-23(17-37-31)14-26-18-39(32(38-30(26)41)43-20-22-4-12-28(34)13-5-22)19-29(40)35-15-21-2-6-24(7-3-21)25-8-10-27(33)11-9-25/h2-13,16-18H,14-15,19-20H2,1H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107481

(CHEMBL153546 | N-(4'-Bromo-biphenyl-4-ylmethyl)-2-...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Br)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29BrFN5O2S/c1-37(17-22-3-7-25(8-4-22)26-9-11-28(33)12-10-26)30(40)20-39-19-27(15-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-5-13-29(34)14-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107501
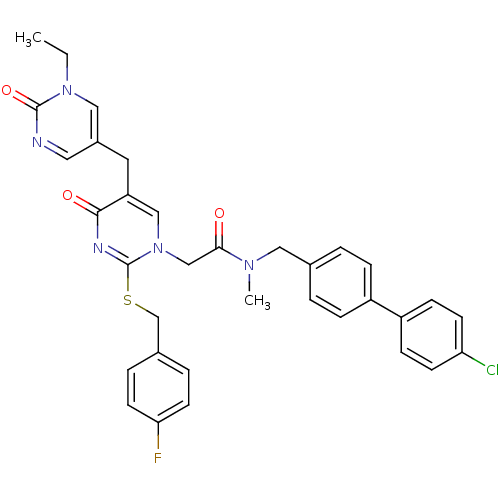
(CHEMBL155227 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CCn1cc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cnc1=O Show InChI InChI=1S/C34H31ClFN5O3S/c1-3-40-19-25(17-37-33(40)44)16-28-20-41(34(38-32(28)43)45-22-24-6-14-30(36)15-7-24)21-31(42)39(2)18-23-4-8-26(9-5-23)27-10-12-29(35)13-11-27/h4-15,17,19-20H,3,16,18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107493
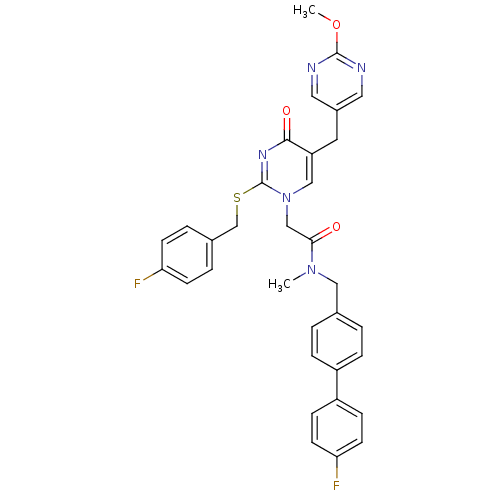
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(F)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H29F2N5O3S/c1-39(18-22-3-7-25(8-4-22)26-9-13-29(35)14-10-26)30(41)20-40-19-27(15-24-16-36-32(43-2)37-17-24)31(42)38-33(40)44-21-23-5-11-28(34)12-6-23/h3-14,16-17,19H,15,18,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107486
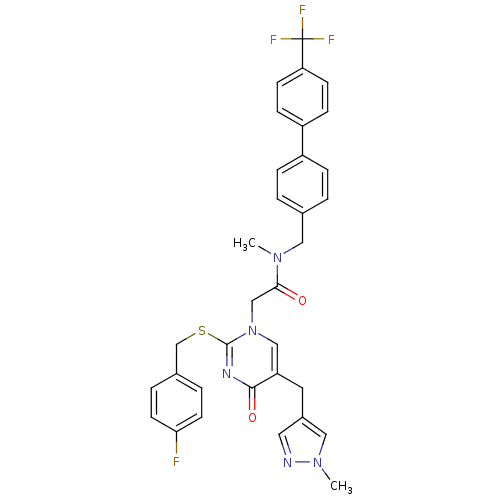
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H29F4N5O2S/c1-40(17-22-3-7-25(8-4-22)26-9-11-28(12-10-26)33(35,36)37)30(43)20-42-19-27(15-24-16-38-41(2)18-24)31(44)39-32(42)45-21-23-5-13-29(34)14-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107494
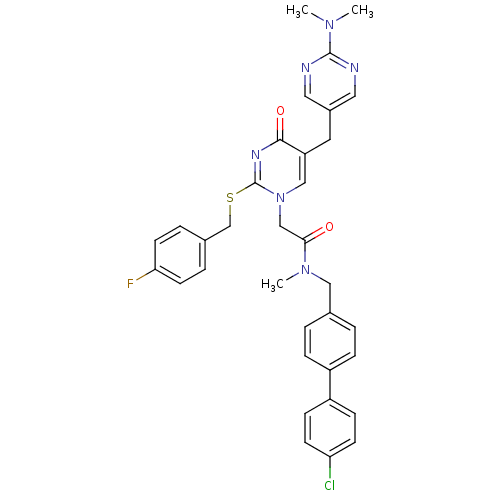
(CHEMBL151095 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(C)c1ncc(Cc2cn(CC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H32ClFN6O2S/c1-40(2)33-37-17-25(18-38-33)16-28-20-42(34(39-32(28)44)45-22-24-6-14-30(36)15-7-24)21-31(43)41(3)19-23-4-8-26(9-5-23)27-10-12-29(35)13-11-27/h4-15,17-18,20H,16,19,21-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107487
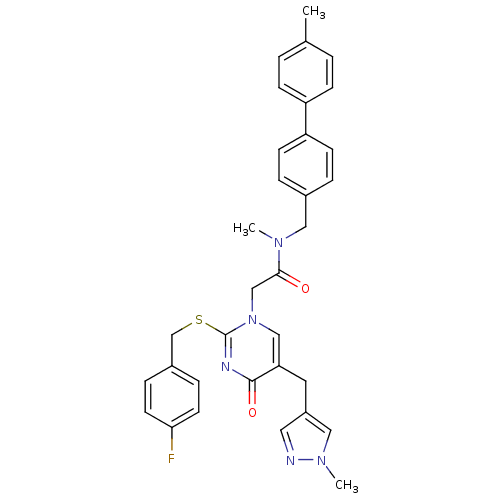
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(C)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H32FN5O2S/c1-23-4-10-27(11-5-23)28-12-6-24(7-13-28)18-37(2)31(40)21-39-20-29(16-26-17-35-38(3)19-26)32(41)36-33(39)42-22-25-8-14-30(34)15-9-25/h4-15,17,19-20H,16,18,21-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107478
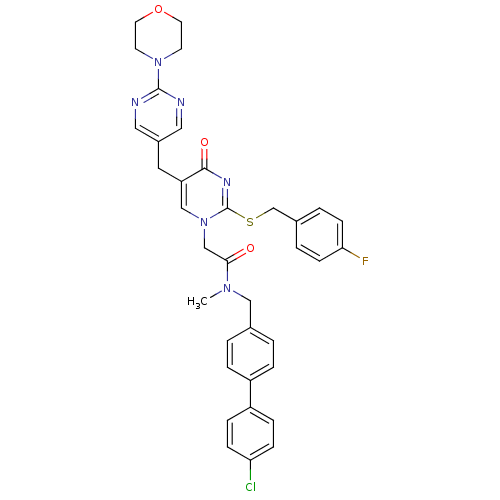
(CHEMBL358052 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(nc2)N2CCOCC2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H34ClFN6O3S/c1-42(21-25-2-6-28(7-3-25)29-8-10-31(37)11-9-29)33(45)23-44-22-30(18-27-19-39-35(40-20-27)43-14-16-47-17-15-43)34(46)41-36(44)48-24-26-4-12-32(38)13-5-26/h2-13,19-20,22H,14-18,21,23-24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107490
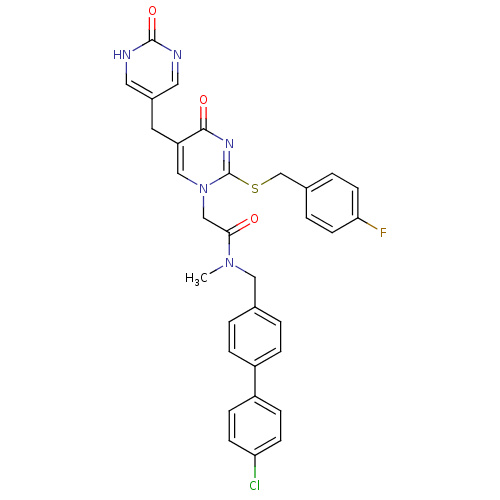
(CHEMBL348030 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(=O)[nH]c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H27ClFN5O3S/c1-38(17-21-2-6-24(7-3-21)25-8-10-27(33)11-9-25)29(40)19-39-18-26(14-23-15-35-31(42)36-16-23)30(41)37-32(39)43-20-22-4-12-28(34)13-5-22/h2-13,15-16,18H,14,17,19-20H2,1H3,(H,35,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107496
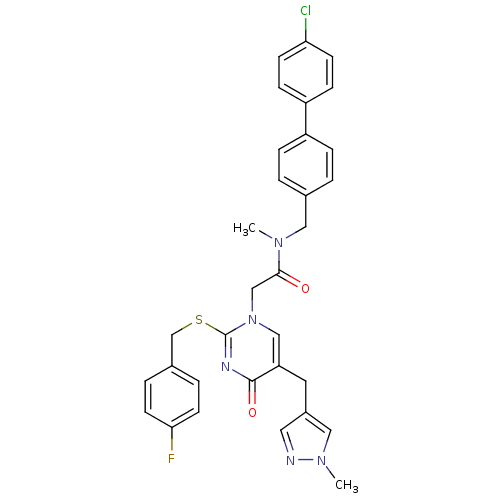
(CHEMBL328023 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29ClFN5O2S/c1-37(17-22-3-7-25(8-4-22)26-9-11-28(33)12-10-26)30(40)20-39-19-27(15-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-5-13-29(34)14-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107488
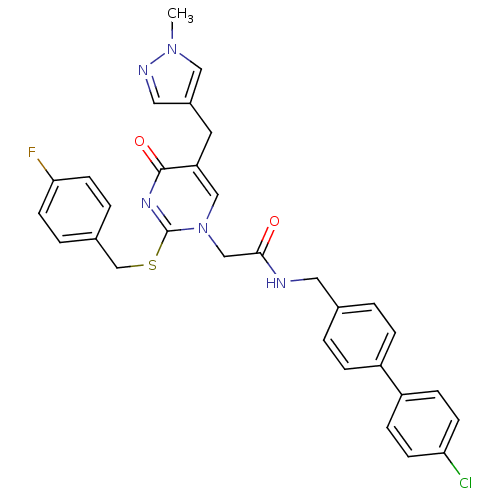
(CHEMBL152387 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES Cn1cc(Cc2cn(CC(=O)NCc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C31H27ClFN5O2S/c1-37-17-23(16-35-37)14-26-18-38(31(36-30(26)40)41-20-22-4-12-28(33)13-5-22)19-29(39)34-15-21-2-6-24(7-3-21)25-8-10-27(32)11-9-25/h2-13,16-18H,14-15,19-20H2,1H3,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107498
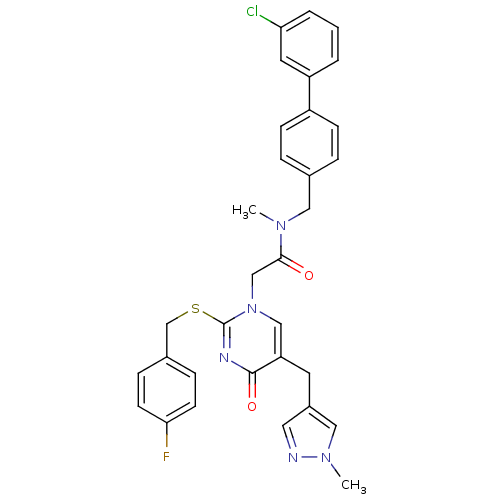
(CHEMBL151061 | N-(3'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1cccc(Cl)c1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29ClFN5O2S/c1-37(17-22-6-10-25(11-7-22)26-4-3-5-28(33)15-26)30(40)20-39-19-27(14-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-8-12-29(34)13-9-23/h3-13,15-16,18-19H,14,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107513
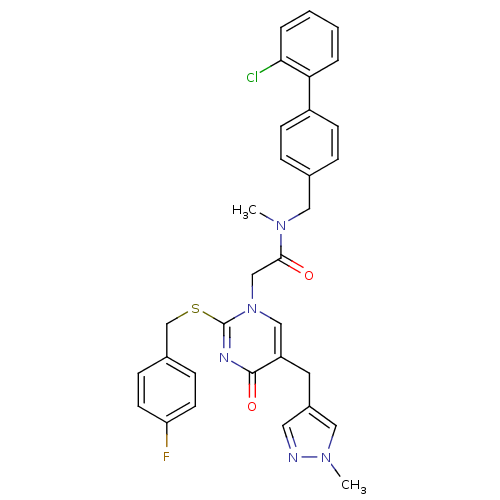
(CHEMBL153579 | N-(2'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1Cl)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29ClFN5O2S/c1-37(17-22-7-11-25(12-8-22)28-5-3-4-6-29(28)33)30(40)20-39-19-26(15-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-9-13-27(34)14-10-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107491
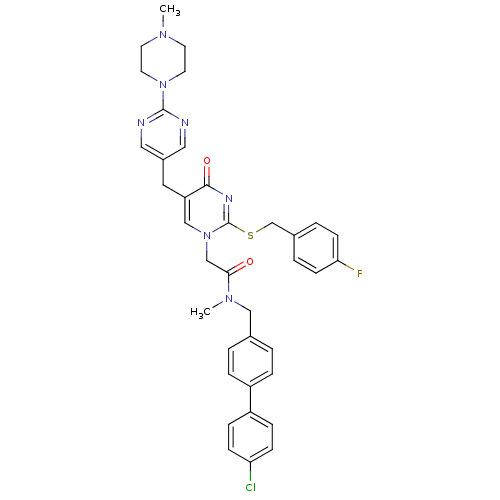
(CHEMBL149644 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(nc2)N2CCN(C)CC2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C37H37ClFN7O2S/c1-43-15-17-45(18-16-43)36-40-20-28(21-41-36)19-31-23-46(37(42-35(31)48)49-25-27-5-13-33(39)14-6-27)24-34(47)44(2)22-26-3-7-29(8-4-26)30-9-11-32(38)12-10-30/h3-14,20-21,23H,15-19,22,24-25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107483
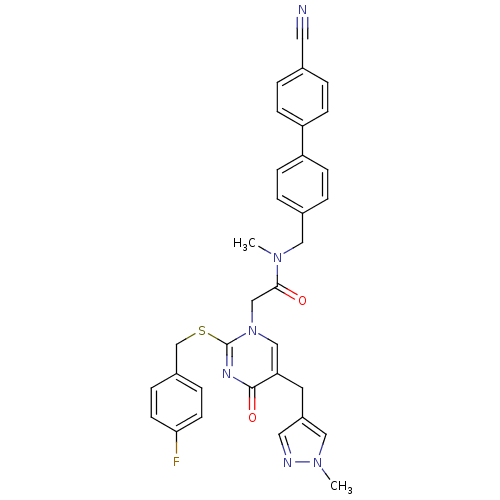
(CHEMBL346439 | N-(4'-Cyano-biphenyl-4-ylmethyl)-2-...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H29FN6O2S/c1-38(18-24-5-11-28(12-6-24)27-9-3-23(16-35)4-10-27)31(41)21-40-20-29(15-26-17-36-39(2)19-26)32(42)37-33(40)43-22-25-7-13-30(34)14-8-25/h3-14,17,19-20H,15,18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107508
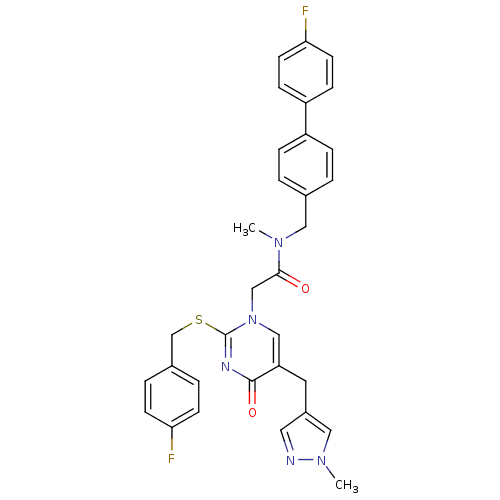
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(F)cc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H29F2N5O2S/c1-37(17-22-3-7-25(8-4-22)26-9-13-29(34)14-10-26)30(40)20-39-19-27(15-24-16-35-38(2)18-24)31(41)36-32(39)42-21-23-5-11-28(33)12-6-23/h3-14,16,18-19H,15,17,20-21H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107489
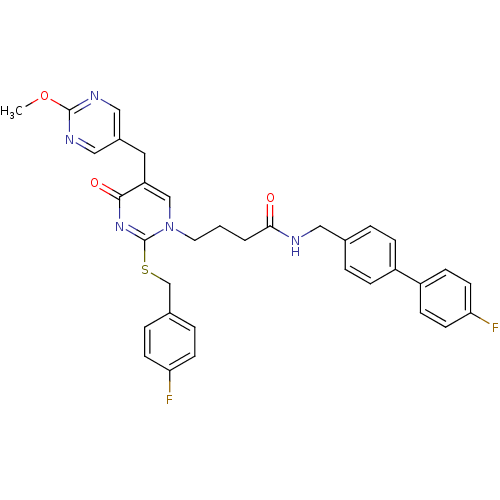
(4-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CCCC(=O)NCc3ccc(cc3)-c3ccc(F)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H31F2N5O3S/c1-44-33-38-19-25(20-39-33)17-28-21-41(34(40-32(28)43)45-22-24-6-12-29(35)13-7-24)16-2-3-31(42)37-18-23-4-8-26(9-5-23)27-10-14-30(36)15-11-27/h4-15,19-21H,2-3,16-18,22H2,1H3,(H,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107511
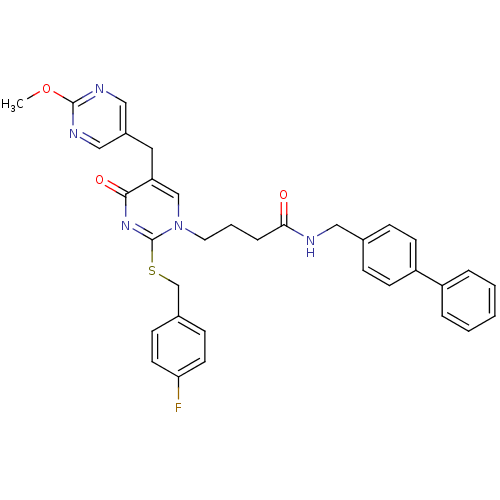
(CHEMBL152705 | N-Biphenyl-4-ylmethyl-4-[2-(4-fluor...)Show SMILES COc1ncc(Cc2cn(CCCC(=O)NCc3ccc(cc3)-c3ccccc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H32FN5O3S/c1-43-33-37-20-26(21-38-33)18-29-22-40(34(39-32(29)42)44-23-25-11-15-30(35)16-12-25)17-5-8-31(41)36-19-24-9-13-28(14-10-24)27-6-3-2-4-7-27/h2-4,6-7,9-16,20-22H,5,8,17-19,23H2,1H3,(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107510
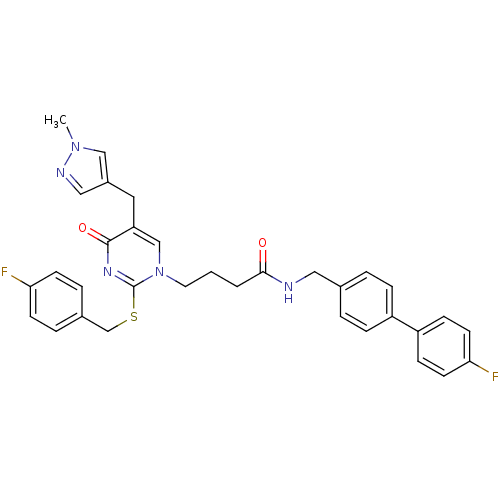
(4-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES Cn1cc(Cc2cn(CCCC(=O)NCc3ccc(cc3)-c3ccc(F)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H31F2N5O2S/c1-39-20-25(19-37-39)17-28-21-40(33(38-32(28)42)43-22-24-6-12-29(34)13-7-24)16-2-3-31(41)36-18-23-4-8-26(9-5-23)27-10-14-30(35)15-11-27/h4-15,19-21H,2-3,16-18,22H2,1H3,(H,36,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107504
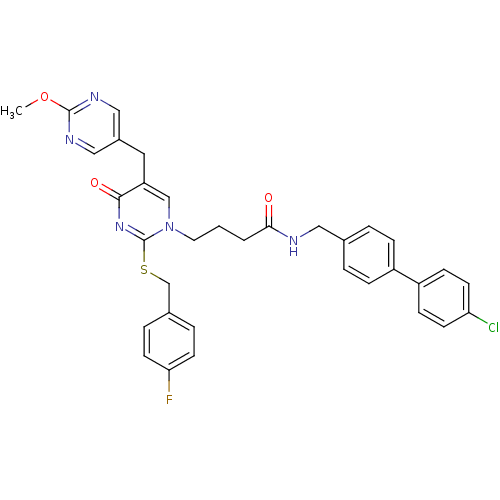
(CHEMBL154817 | N-(4'-Chloro-biphenyl-4-ylmethyl)-4...)Show SMILES COc1ncc(Cc2cn(CCCC(=O)NCc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C34H31ClFN5O3S/c1-44-33-38-19-25(20-39-33)17-28-21-41(34(40-32(28)43)45-22-24-6-14-30(36)15-7-24)16-2-3-31(42)37-18-23-4-8-26(9-5-23)27-10-12-29(35)13-11-27/h4-15,19-21H,2-3,16-18,22H2,1H3,(H,37,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107503
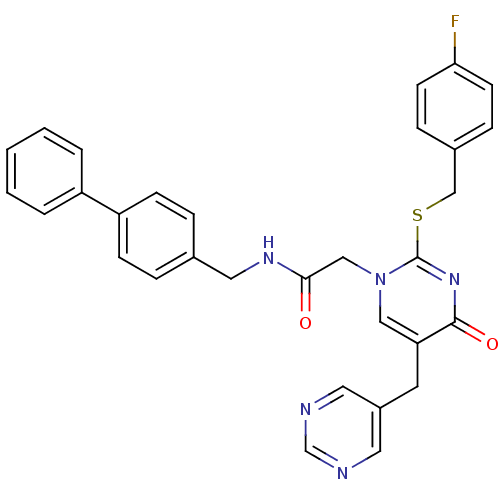
(CHEMBL153681 | N-Biphenyl-4-ylmethyl-2-[2-(4-fluor...)Show SMILES Fc1ccc(CSc2nc(=O)c(Cc3cncnc3)cn2CC(=O)NCc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C31H26FN5O2S/c32-28-12-8-23(9-13-28)20-40-31-36-30(39)27(14-24-15-33-21-34-16-24)18-37(31)19-29(38)35-17-22-6-10-26(11-7-22)25-4-2-1-3-5-25/h1-13,15-16,18,21H,14,17,19-20H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107506
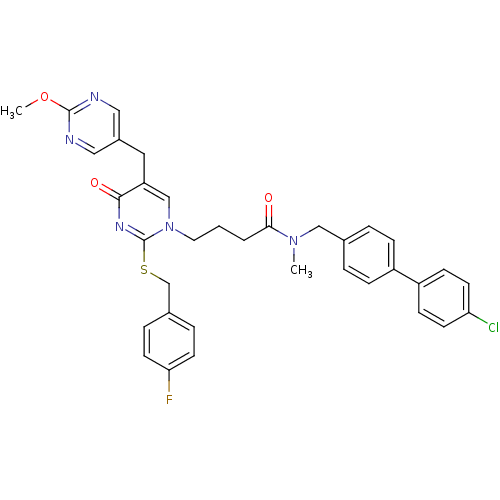
(CHEMBL153246 | N-(4'-Chloro-biphenyl-4-ylmethyl)-4...)Show SMILES COc1ncc(Cc2cn(CCCC(=O)N(C)Cc3ccc(cc3)-c3ccc(Cl)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C35H33ClFN5O3S/c1-41(21-24-5-9-27(10-6-24)28-11-13-30(36)14-12-28)32(43)4-3-17-42-22-29(18-26-19-38-34(45-2)39-20-26)33(44)40-35(42)46-23-25-7-15-31(37)16-8-25/h5-16,19-20,22H,3-4,17-18,21,23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107482
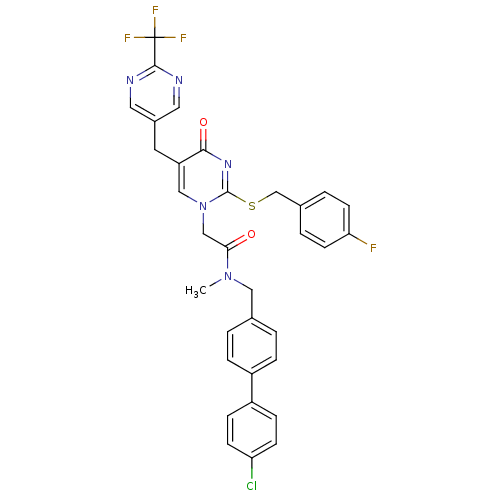
(CHEMBL348431 | N-(4'-Chloro-biphenyl-4-ylmethyl)-2...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)Cn1cc(Cc2cnc(nc2)C(F)(F)F)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H26ClF4N5O2S/c1-42(17-21-2-6-24(7-3-21)25-8-10-27(34)11-9-25)29(44)19-43-18-26(14-23-15-39-31(40-16-23)33(36,37)38)30(45)41-32(43)46-20-22-4-12-28(35)13-5-22/h2-13,15-16,18H,14,17,19-20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107502
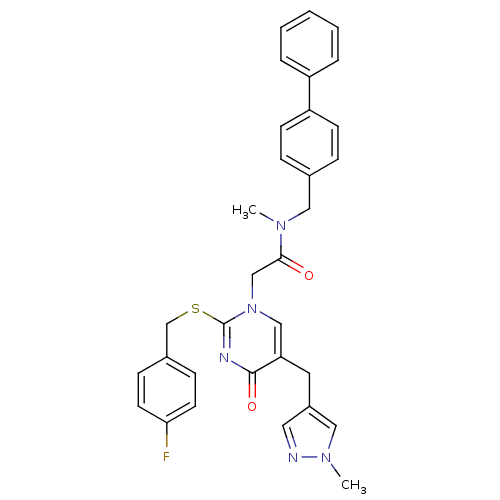
(CHEMBL150336 | N-Biphenyl-4-ylmethyl-2-[2-(4-fluor...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H30FN5O2S/c1-36(18-23-8-12-27(13-9-23)26-6-4-3-5-7-26)30(39)21-38-20-28(16-25-17-34-37(2)19-25)31(40)35-32(38)41-22-24-10-14-29(33)15-11-24/h3-15,17,19-20H,16,18,21-22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107484
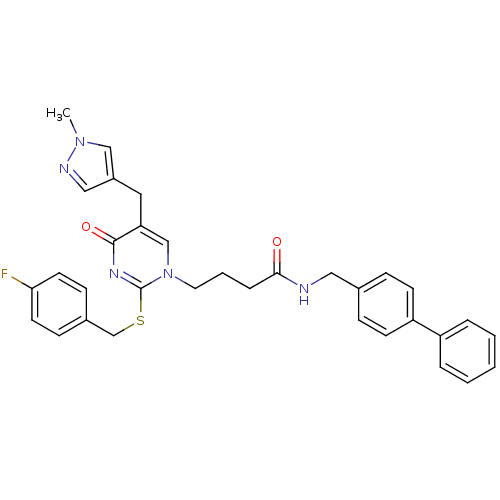
(CHEMBL150823 | N-Biphenyl-4-ylmethyl-4-[2-(4-fluor...)Show SMILES Cn1cc(Cc2cn(CCCC(=O)NCc3ccc(cc3)-c3ccccc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C33H32FN5O2S/c1-38-21-26(20-36-38)18-29-22-39(33(37-32(29)41)42-23-25-11-15-30(34)16-12-25)17-5-8-31(40)35-19-24-9-13-28(14-10-24)27-6-3-2-4-7-27/h2-4,6-7,9-16,20-22H,5,8,17-19,23H2,1H3,(H,35,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
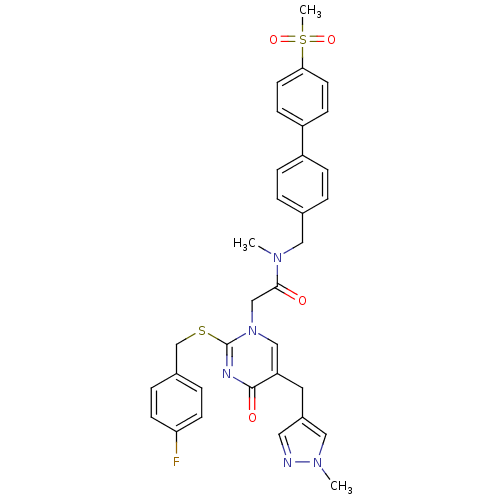
(Homo sapiens (Human)) | BDBM50107500
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C33H32FN5O4S2/c1-37(18-23-4-8-26(9-5-23)27-10-14-30(15-11-27)45(3,42)43)31(40)21-39-20-28(16-25-17-35-38(2)19-25)32(41)36-33(39)44-22-24-6-12-29(34)13-7-24/h4-15,17,19-20H,16,18,21-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107497
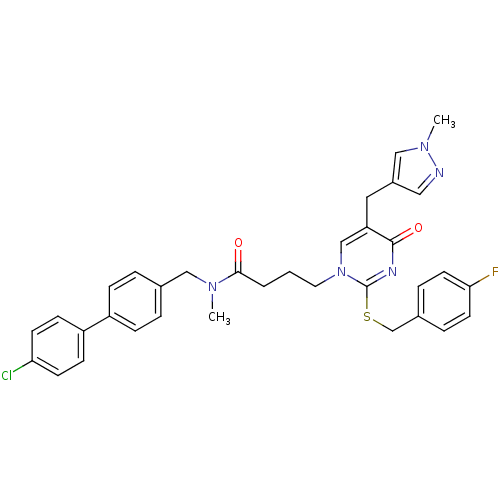
(CHEMBL273524 | N-(4'-Chloro-biphenyl-4-ylmethyl)-4...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(Cl)cc1)C(=O)CCCn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H33ClFN5O2S/c1-39(20-24-5-9-27(10-6-24)28-11-13-30(35)14-12-28)32(42)4-3-17-41-22-29(18-26-19-37-40(2)21-26)33(43)38-34(41)44-23-25-7-15-31(36)16-8-25/h5-16,19,21-22H,3-4,17-18,20,23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107499
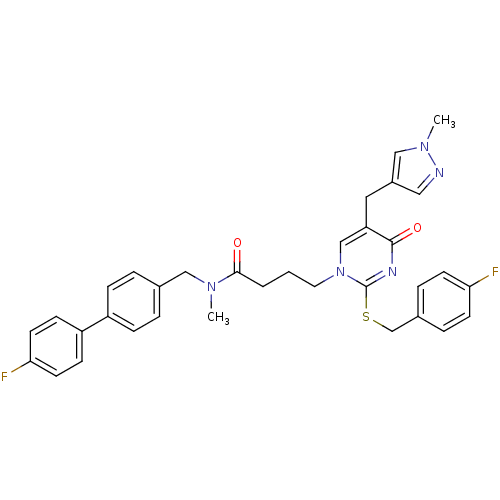
(4-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES CN(Cc1ccc(cc1)-c1ccc(F)cc1)C(=O)CCCn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C34H33F2N5O2S/c1-39(20-24-5-9-27(10-6-24)28-11-15-31(36)16-12-28)32(42)4-3-17-41-22-29(18-26-19-37-40(2)21-26)33(43)38-34(41)44-23-25-7-13-30(35)14-8-25/h5-16,19,21-22H,3-4,17-18,20,23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107509

(CHEMBL153701 | N-Biphenyl-4-ylmethyl-2-[2-(4-fluor...)Show SMILES CN(Cc1ccc(cc1)-c1ccccc1)C(=O)Cn1cc(Cc2cncnc2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C32H28FN5O2S/c1-37(18-23-7-11-27(12-8-23)26-5-3-2-4-6-26)30(39)20-38-19-28(15-25-16-34-22-35-17-25)31(40)36-32(38)41-21-24-9-13-29(33)14-10-24/h2-14,16-17,19,22H,15,18,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107492
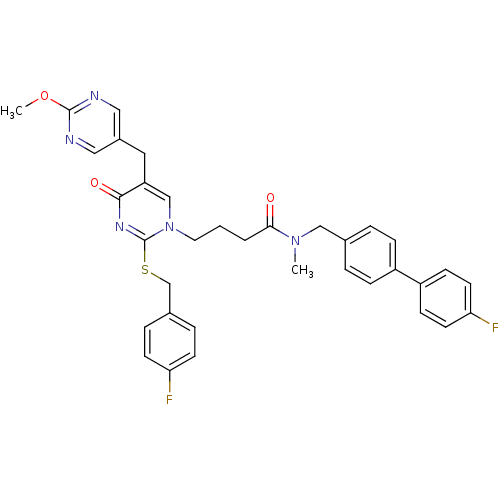
(4-[2-(4-Fluoro-benzylsulfanyl)-5-(2-methoxy-pyrimi...)Show SMILES COc1ncc(Cc2cn(CCCC(=O)N(C)Cc3ccc(cc3)-c3ccc(F)cc3)c(SCc3ccc(F)cc3)nc2=O)cn1 Show InChI InChI=1S/C35H33F2N5O3S/c1-41(21-24-5-9-27(10-6-24)28-11-15-31(37)16-12-28)32(43)4-3-17-42-22-29(18-26-19-38-34(45-2)39-20-26)33(44)40-35(42)46-23-25-7-13-30(36)14-8-25/h5-16,19-20,22H,3-4,17-18,21,23H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107479
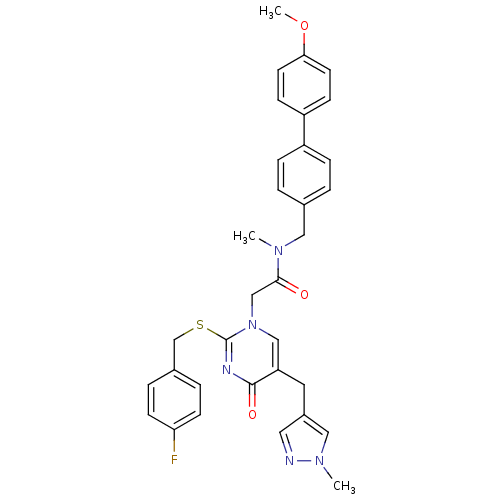
(2-[2-(4-Fluoro-benzylsulfanyl)-5-(1-methyl-1H-pyra...)Show SMILES COc1ccc(cc1)-c1ccc(CN(C)C(=O)Cn2cc(Cc3cnn(C)c3)c(=O)nc2SCc2ccc(F)cc2)cc1 Show InChI InChI=1S/C33H32FN5O3S/c1-37(18-23-4-8-26(9-5-23)27-10-14-30(42-3)15-11-27)31(40)21-39-20-28(16-25-17-35-38(2)19-25)32(41)36-33(39)43-22-24-6-12-29(34)13-7-24/h4-15,17,19-20H,16,18,21-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107485
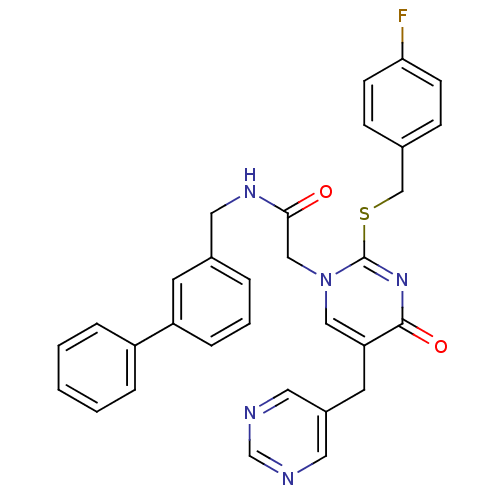
(CHEMBL345781 | N-Biphenyl-3-ylmethyl-2-[2-(4-fluor...)Show SMILES Fc1ccc(CSc2nc(=O)c(Cc3cncnc3)cn2CC(=O)NCc2cccc(c2)-c2ccccc2)cc1 Show InChI InChI=1S/C31H26FN5O2S/c32-28-11-9-22(10-12-28)20-40-31-36-30(39)27(14-24-15-33-21-34-16-24)18-37(31)19-29(38)35-17-23-5-4-8-26(13-23)25-6-2-1-3-7-25/h1-13,15-16,18,21H,14,17,19-20H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50107495
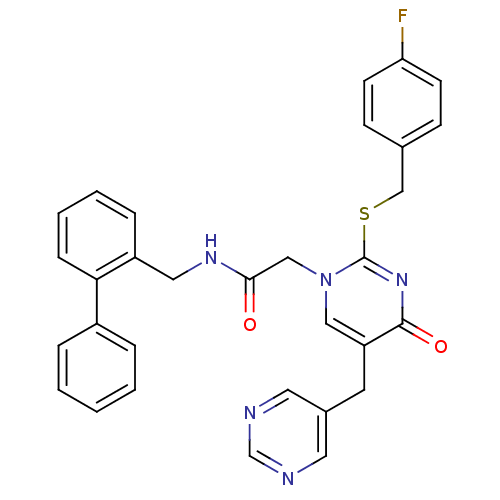
(CHEMBL153415 | N-Biphenyl-2-ylmethyl-2-[2-(4-fluor...)Show SMILES Fc1ccc(CSc2nc(=O)c(Cc3cncnc3)cn2CC(=O)NCc2ccccc2-c2ccccc2)cc1 Show InChI InChI=1S/C31H26FN5O2S/c32-27-12-10-22(11-13-27)20-40-31-36-30(39)26(14-23-15-33-21-34-16-23)18-37(31)19-29(38)35-17-25-8-4-5-9-28(25)24-6-2-1-3-7-24/h1-13,15-16,18,21H,14,17,19-20H2,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 (Lp-PLA2). |
Bioorg Med Chem Lett 12: 51-5 (2001)
BindingDB Entry DOI: 10.7270/Q2862FR5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data