

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50133259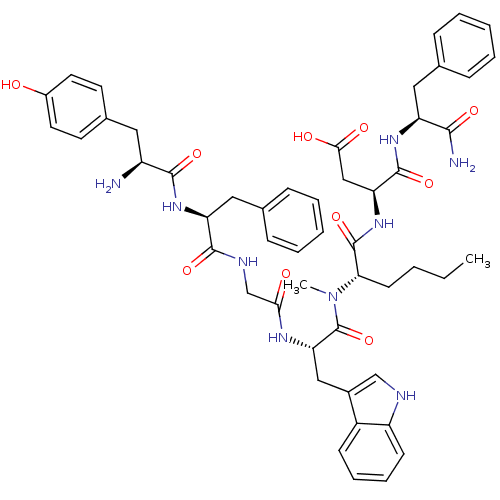 (CHEMBL430298 | H-Tyr-D-Phe-Gly-D-Trp-N-MeNle-Asp-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50133267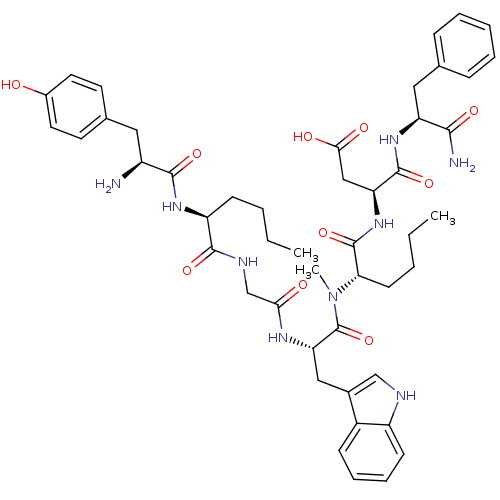 (CHEMBL132314 | H-Tyr-D-Nle-Gly-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21141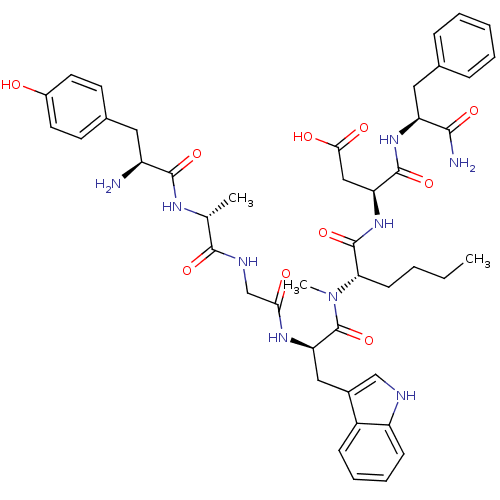 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21136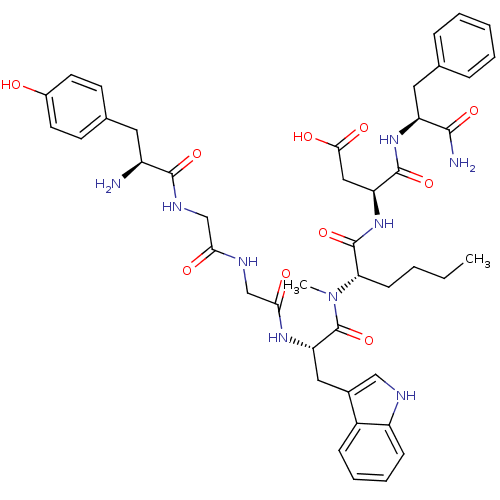 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50133267 (CHEMBL132314 | H-Tyr-D-Nle-Gly-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50133259 (CHEMBL430298 | H-Tyr-D-Phe-Gly-D-Trp-N-MeNle-Asp-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50133268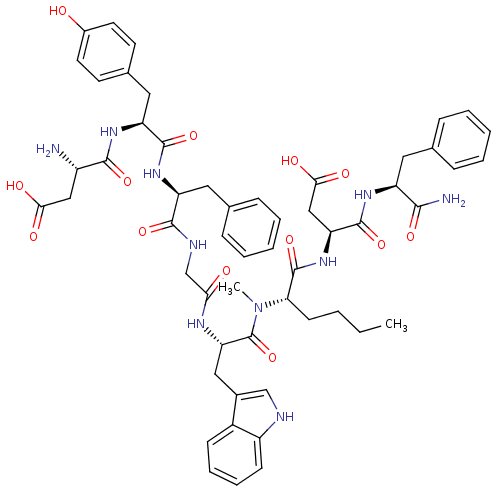 (CHEMBL265595 | H-Asp-Tyr-D-Phe-Gly-Trp-N-MeNle-Asp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50133265 (CHEMBL411201 | H-Tyr-D-Phe-Glu-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor (CCK-B) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133259 (CHEMBL430298 | H-Tyr-D-Phe-Gly-D-Trp-N-MeNle-Asp-P...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50133265 (CHEMBL411201 | H-Tyr-D-Phe-Glu-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133267 (CHEMBL132314 | H-Tyr-D-Nle-Gly-Trp-N-MeNle-Asp-Phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133265 (CHEMBL411201 | H-Tyr-D-Phe-Glu-Trp-N-MeNle-Asp-Phe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50133268 (CHEMBL265595 | H-Asp-Tyr-D-Phe-Gly-Trp-N-MeNle-Asp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50133259 (CHEMBL430298 | H-Tyr-D-Phe-Gly-D-Trp-N-MeNle-Asp-P...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50133268 (CHEMBL265595 | H-Asp-Tyr-D-Phe-Gly-Trp-N-MeNle-Asp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50133267 (CHEMBL132314 | H-Tyr-D-Nle-Gly-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133268 (CHEMBL265595 | H-Asp-Tyr-D-Phe-Gly-Trp-N-MeNle-Asp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50133265 (CHEMBL411201 | H-Tyr-D-Phe-Glu-Trp-N-MeNle-Asp-Phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type A receptor (CCK-A) receptor was determined | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 using [3H]- [p-CIPhe4] DPDPE as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133257 (CHEMBL334369 | [(2S,3R)-TMT1]DPDPE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 using [3H]- [p-CIPhe4] DPDPE as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50027070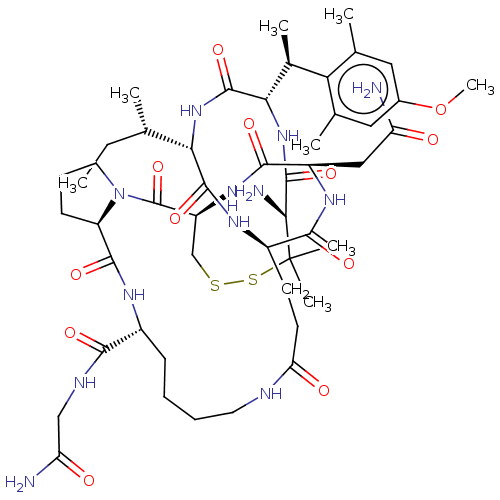 (CHEMBL2369136) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50133272 (CHEMBL406351 | [D-Pen1,Glu4,Lys8]OT) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50133258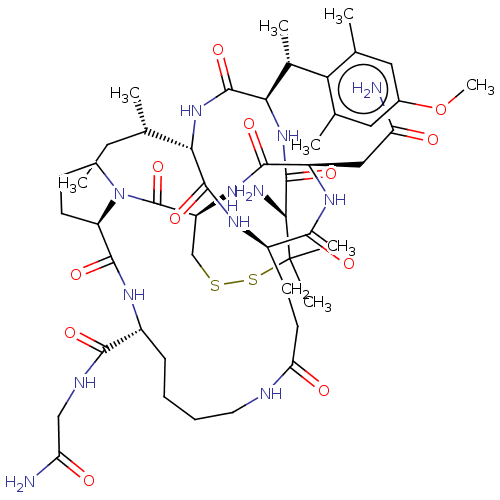 (CHEMBL2369135 | [D-Pen1,(2S,3R)-p-MeOTMT2,Glu4,Lys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133264 (CHEMBL133719 | [(2S,3S)-TMT1]DPDPE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 using [3H]- [p-CIPhe4] DPDPE as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133264 (CHEMBL133719 | [(2S,3S)-TMT1]DPDPE) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50133261 (CHEMBL134652 | [(2R,3R)-TMT1]DPDPE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 using [3H]- [p-CIPhe4] DPDPE as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133257 (CHEMBL334369 | [(2S,3R)-TMT1]DPDPE) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50027082 (CHEMBL2370761) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50027076 (CHEMBL2370768) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards oxytocin receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50133261 (CHEMBL134652 | [(2R,3R)-TMT1]DPDPE) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 using [3H]- CTOP as radioligand | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50133264 (CHEMBL133719 | [(2S,3S)-TMT1]DPDPE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor delta 1 in functional assa... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50029747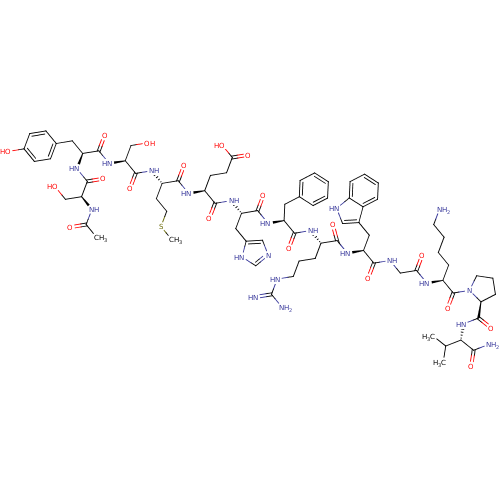 ((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 4 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50017181 (CHEMBL441738) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 1 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor delta 1 in functional assa... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50133264 (CHEMBL133719 | [(2S,3S)-TMT1]DPDPE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor mu 1 in functional assay,G... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50133261 (CHEMBL134652 | [(2R,3R)-TMT1]DPDPE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor mu 1 in functional assay,G... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50133273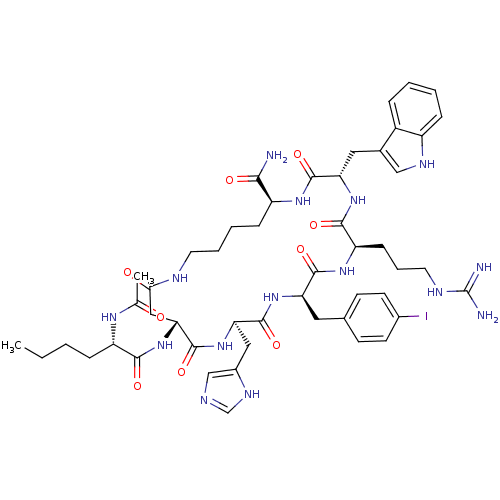 (Ac-Nle4-c[Asp5,D-Phe(pI)7,Lys10]R-MSH(4-10)-NH2 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 1 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor mu 1 in functional assay,G... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50121268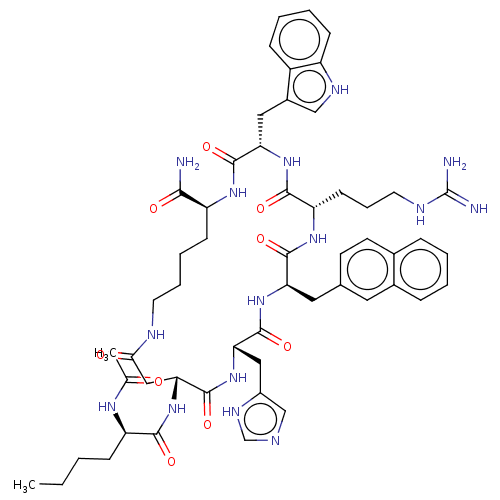 (21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 1 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50027084 (Melatonan) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 4 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50029747 ((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 3 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50017181 (CHEMBL441738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 3 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50029747 ((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration required for the biological activity against human Melanocortin 1 receptor | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |