

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123752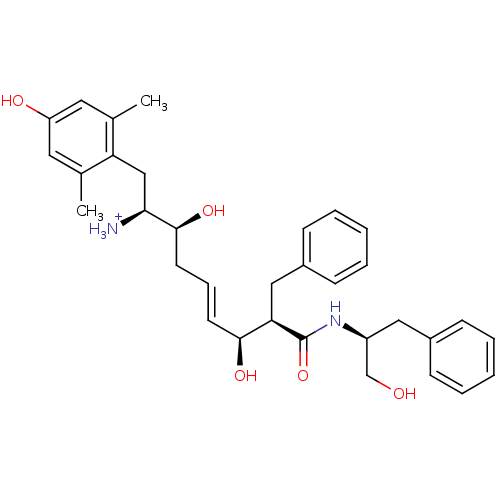 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123763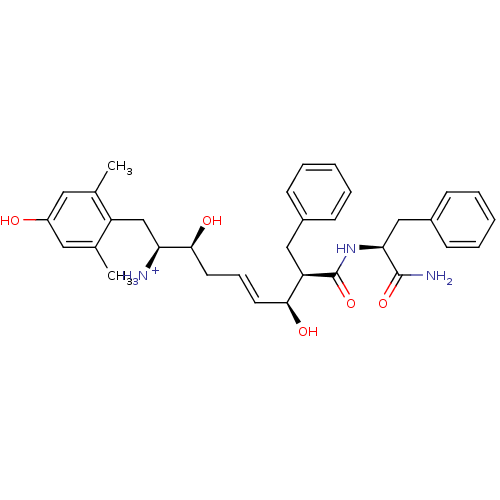 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123756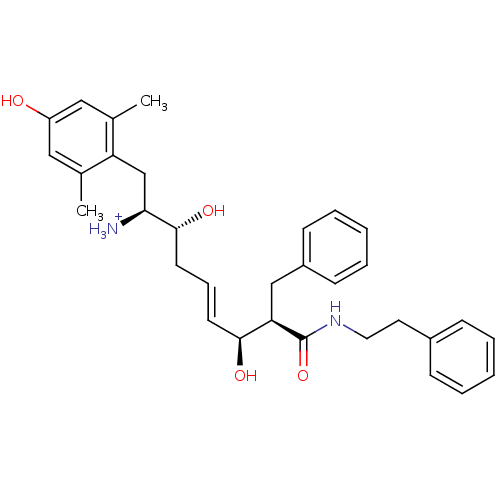 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123754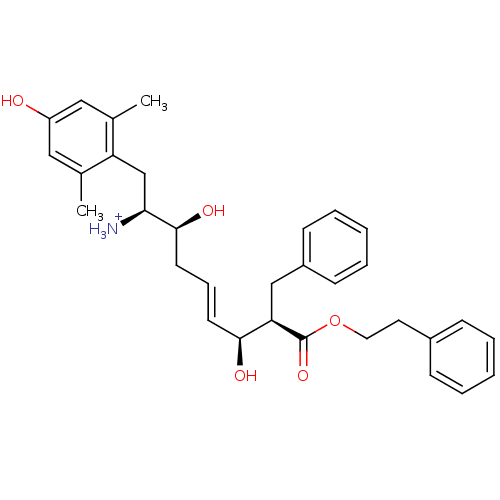 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123750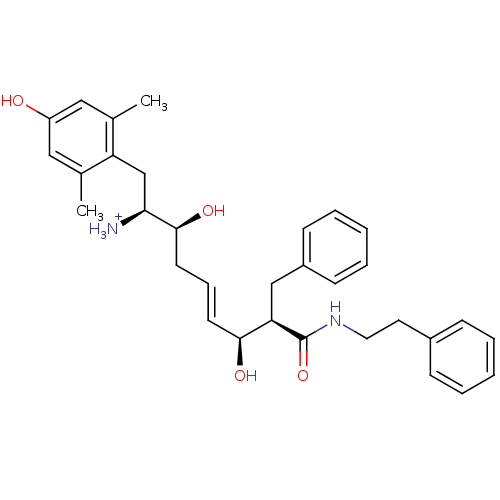 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123751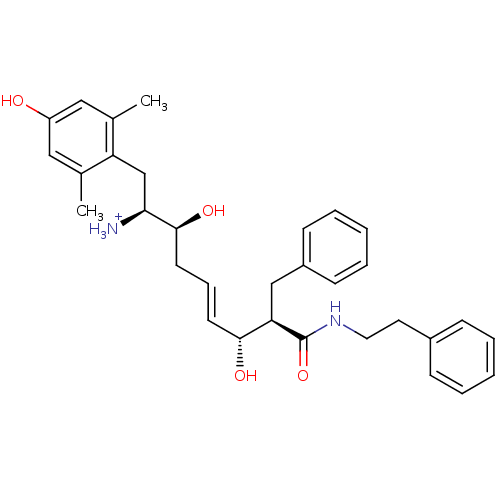 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123759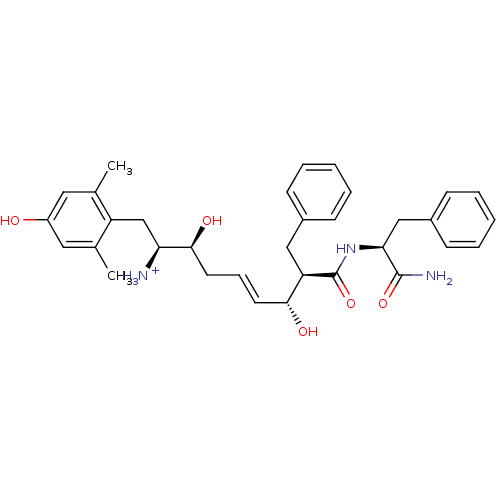 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123762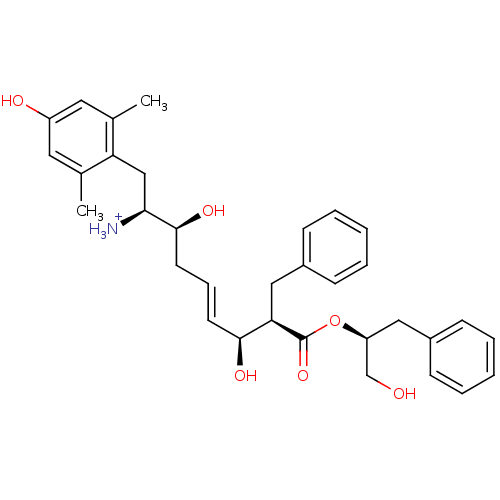 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123760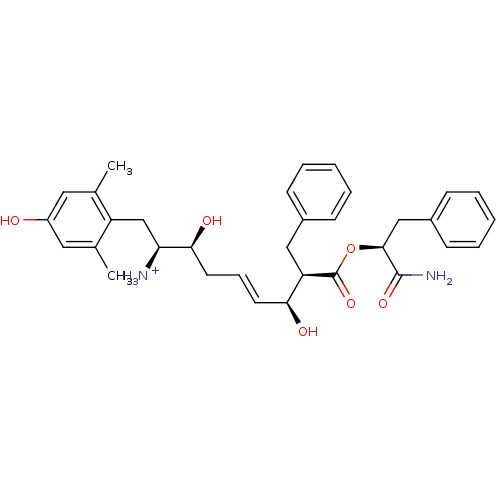 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123757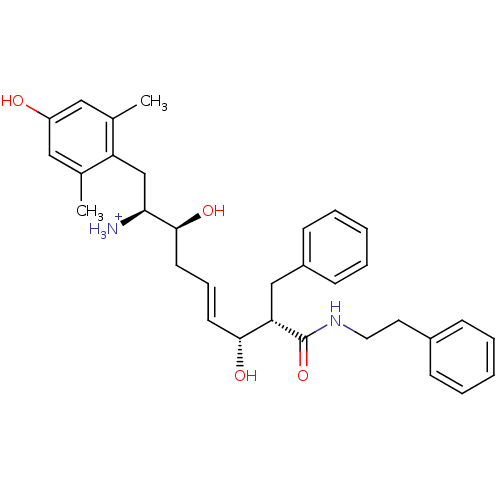 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123755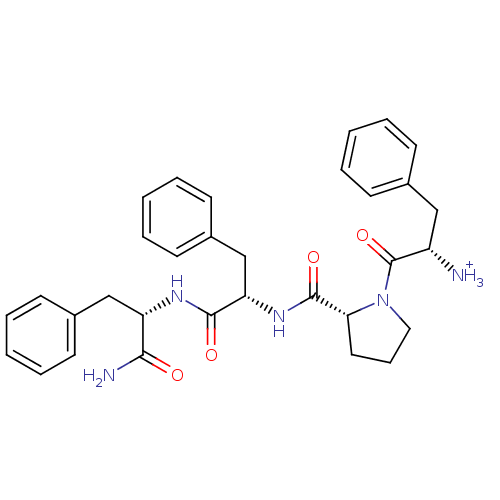 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123761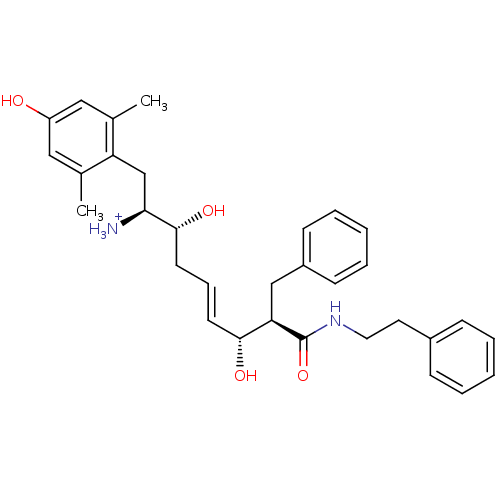 ((E)-(1S,2R,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123753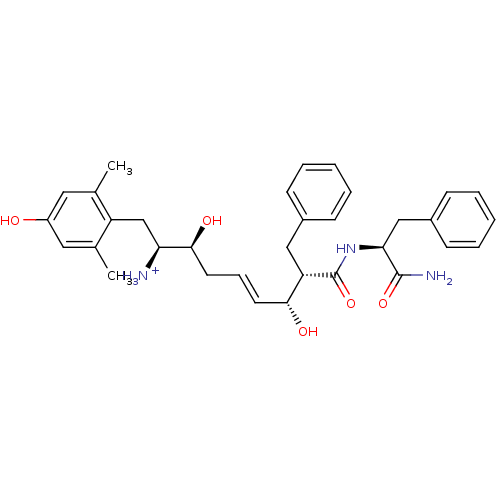 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123758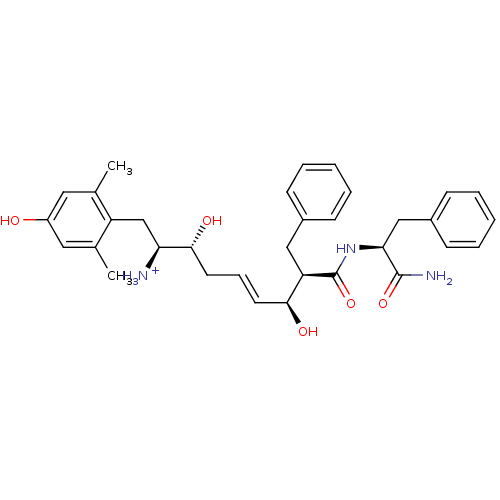 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123749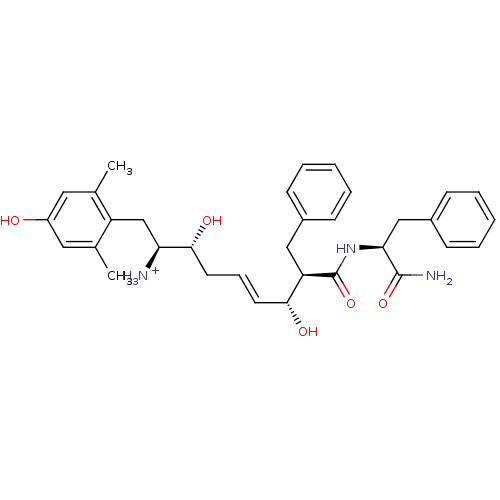 ((E)-(1S,2R,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human Opioid receptor mu 1 expressing CHO cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123758 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123762 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123760 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123759 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123763 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123749 ((E)-(1S,2R,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123751 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123759 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123763 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123750 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123758 ((E)-(1S,2R,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123753 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123756 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123752 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123754 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123757 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123751 ((E)-(1S,2S,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123761 ((E)-(1S,2R,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123761 ((E)-(1S,2R,6R,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123752 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123749 ((E)-(1S,2R,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123762 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123750 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123757 ((E)-(1S,2S,6R,7S)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123756 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123760 ((E)-(1S,2S,6S,7R)-7-((S)-1-Carbamoyl-2-phenyl-etho...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123753 ((E)-(1S,2S,6R,7S)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123754 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123755 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of 3H-U-69,593 from Opioid receptor kappa 1 in guinea pig cerebellum preparation | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123755 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human Opioid receptor delta 1 epressing HEK293 cells | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123755 (2-{(R)-2-[1-((S)-(S)-1-Carbamoyl-2-phenyl-ethylcar...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Compound tested at 10 uM for the ability to induce Opioid receptor mu 1-mediated binding of [35S]GTP-gamma-S to G proteins in CHO membrane preparatio... | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123759 ((E)-(1S,2S,6R,7R)-7-((S)-1-Carbamoyl-2-phenyl-ethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Compound tested at 10 uM for the ability to induce Opioid receptor mu 1-mediated binding of [35S]GTP-gamma-S to G proteins in CHO membrane preparatio... | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123756 ((E)-(1S,2R,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Compound tested at 10 uM for the ability to induce Opioid receptor mu 1-mediated binding of [35S]GTP-gamma-S to G proteins in CHO membrane preparatio... | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50123754 ((E)-(1S,2S,6S,7R)-2,6-Dihydroxy-1-(4-hydroxy-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Compound tested at 10 uM for the ability to induce Opioid receptor mu 1-mediated binding of [35S]GTP-gamma-S to G proteins in CHO membrane preparatio... | J Med Chem 46: 677-80 (2003) Article DOI: 10.1021/jm025608s BindingDB Entry DOI: 10.7270/Q2959J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||