

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50209670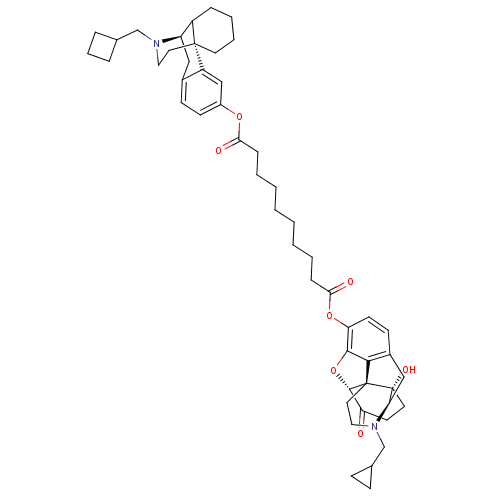 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027231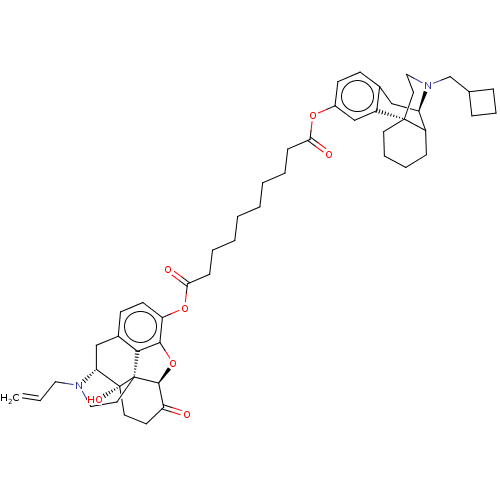 (CHEMBL2113275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human opioid gamma receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50209670 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50209671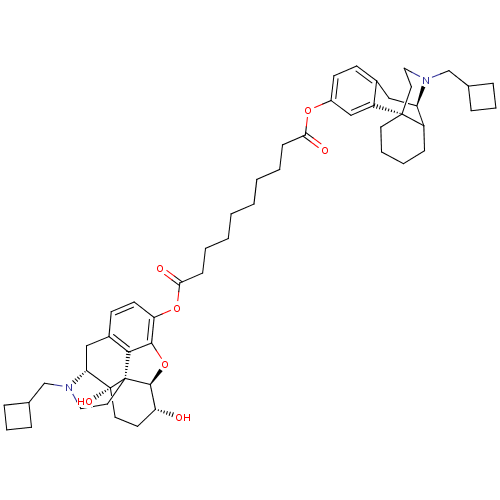 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027231 (CHEMBL2113275) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human opioid gamma receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50209671 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human opioid gamma receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50209670 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50209671 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50027231 (CHEMBL2113275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human opioid delta receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50209671 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027231 (CHEMBL2113275) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50209670 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as inhibition of DAGO-stimulated [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human opioid kappa receptor expressed in CHO cells assessed as inhibition of U-50488-stimulated [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human opioid kappa receptor expressed in CHO cells assessed as inhibition of U-50488-stimulated [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50209671 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human opioid kappa receptor expressed in CHO cells assessed as inhibition of U-50488-stimulated [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50180185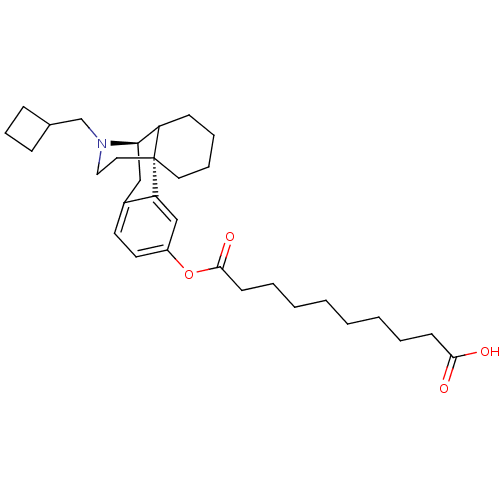 (10-{[(1R,9R)-17-(cyclobutylmethyl)-17-azatetracycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50180185 (10-{[(1R,9R)-17-(cyclobutylmethyl)-17-azatetracycl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50027231 (CHEMBL2113275) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027231 (CHEMBL2113275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50209671 ((5alpha,6alpha)-17-(cyclobutylmethyl)-6,14-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50209670 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50209670 (17-(cyclopropylmethyl)morphinan-3-yl(5alpha)-17-(c...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid gamma receptor expressed in CHO cells assessed as stimulation of [35S]GTP-gamma-S binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||