

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304170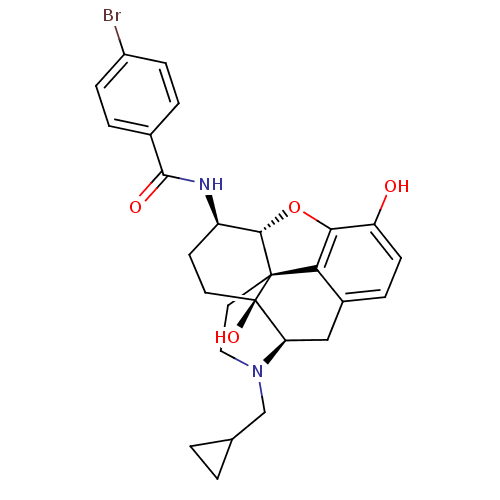 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50378578 (CHEMBL1627119) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304175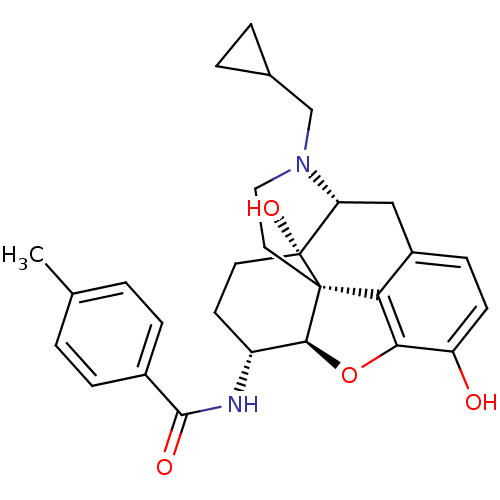 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304174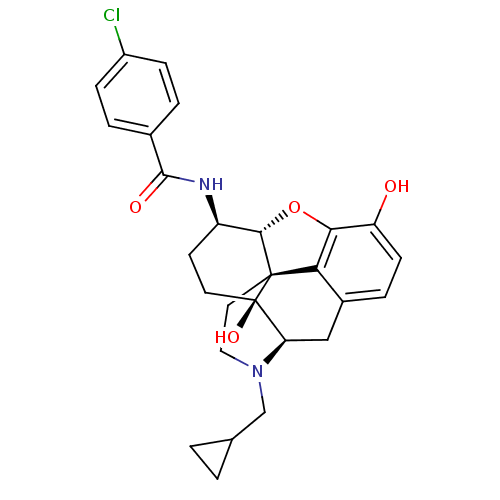 (4-chloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304170 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304173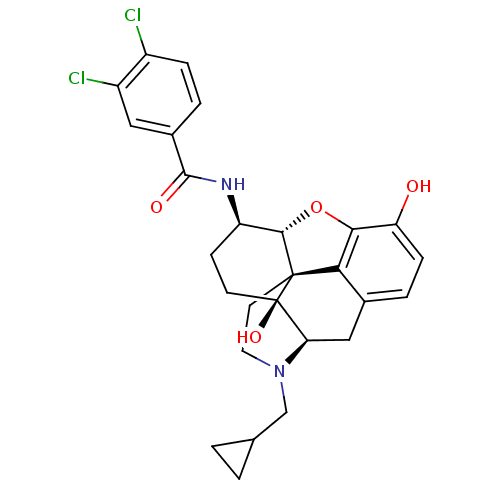 (3,4-dichloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304176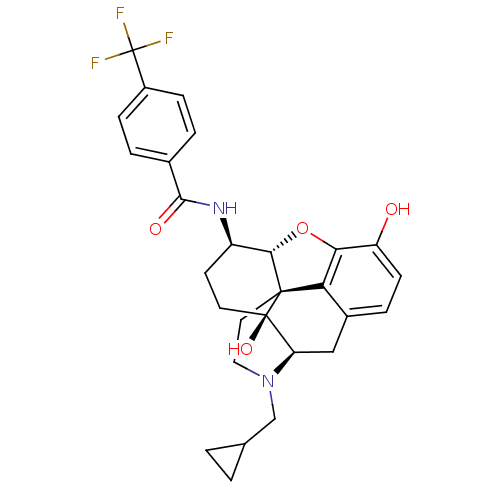 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304171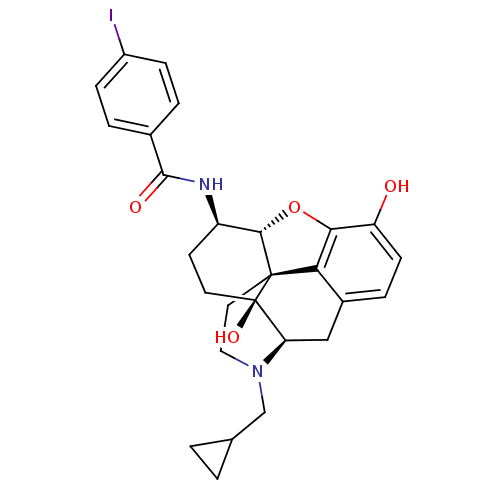 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304176 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304173 (3,4-dichloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304174 (4-chloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmeth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50304176 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor expressed in HEK293 cells assessed as inhibition of compound 16-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50159165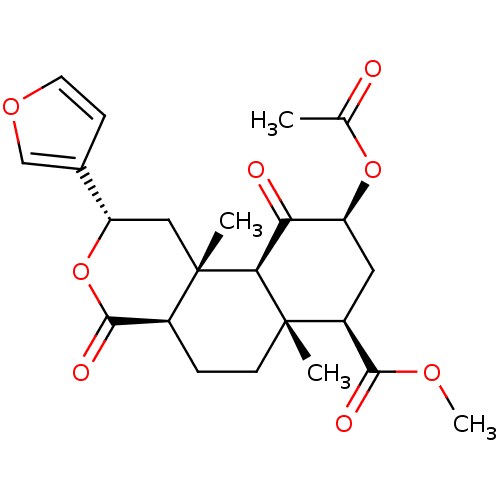 ((2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(fura...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304171 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304170 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045776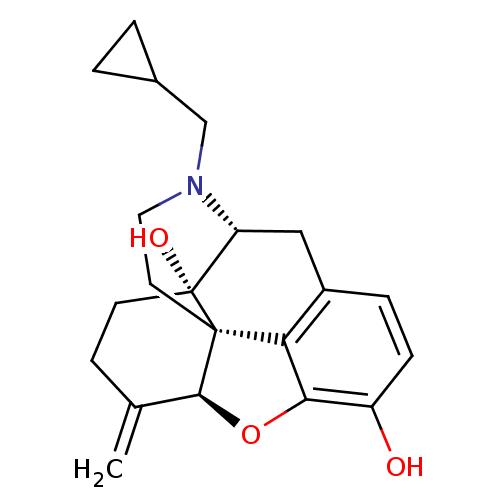 (CHEMBL982 | JF-1 | NALMEFENE | Nalmetrene | ORF-11...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045776 (CHEMBL982 | JF-1 | NALMEFENE | Nalmetrene | ORF-11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304173 (3,4-dichloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304171 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304170 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304176 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304174 (4-chloro-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of compound 11-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50304176 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NOP expressed in HEK293 cells assessed as inhibition of compound 15-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304171 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304176 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304170 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045776 (CHEMBL982 | JF-1 | NALMEFENE | Nalmetrene | ORF-11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor expressed in HEK293 cells by visible spectrophotometry | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000787 ((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at delta opioid receptor expressed in HEK293 cells assessed as inhibition of compound 14-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of compound 11-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at mu opioid receptor expressed in HEK293 cells assessed as inhibition of compound 11-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50304175 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NOP expressed in HEK293 cells assessed as inhibition of compound 15-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50304170 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NOP expressed in HEK293 cells assessed as inhibition of compound 15-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50304171 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NOP expressed in HEK293 cells assessed as inhibition of compound 15-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50304172 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Human BioMolecular Research Institute Curated by ChEMBL | Assay Description Antagonist activity at NOP expressed in HEK293 cells assessed as inhibition of compound 15-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 6671-81 (2009) Article DOI: 10.1016/j.bmc.2009.07.069 BindingDB Entry DOI: 10.7270/Q2PK0H37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |