

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Trypsin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Plasmin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit tissue-type plasminogen activator (t-PA) was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50120225 (2-{[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Coagulation factor X was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120233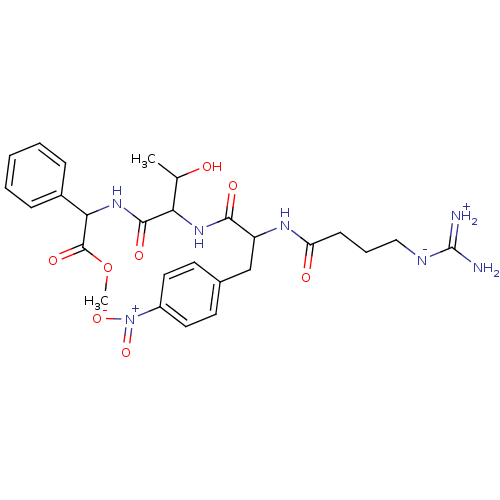 (CHEMBL321268 | {2-[2-(4-Guanidino-butyrylamino)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Coagulation factor X was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120228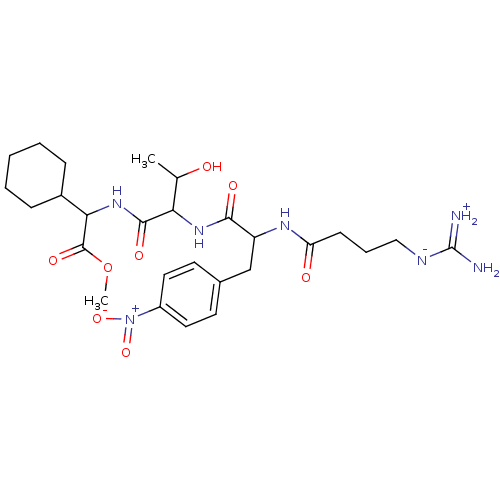 (CHEMBL111195 | Cyclohexyl-{2-[2-(4-guanidino-butyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120231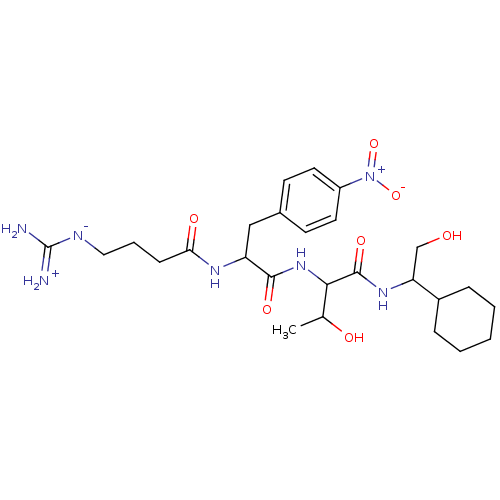 (CHEMBL419202 | N-(1-Cyclohexyl-2-hydroxy-ethyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120227 (2-[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Plasmin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Tissue-type plasminogen activator (t-PA) was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120229 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-(4-nitro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120232 (CHEMBL327093 | Cyclohexyl-{2-[2-(4-guanidino-butyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120224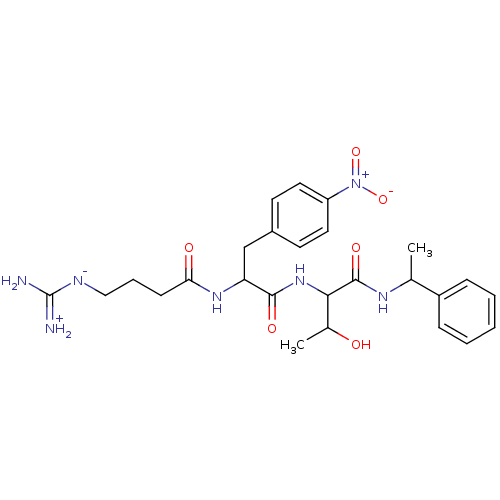 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-(4-nitro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120229 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-(4-nitro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory constant of thrombin catalytic activity of the compound was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120224 ((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-(4-nitro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50120226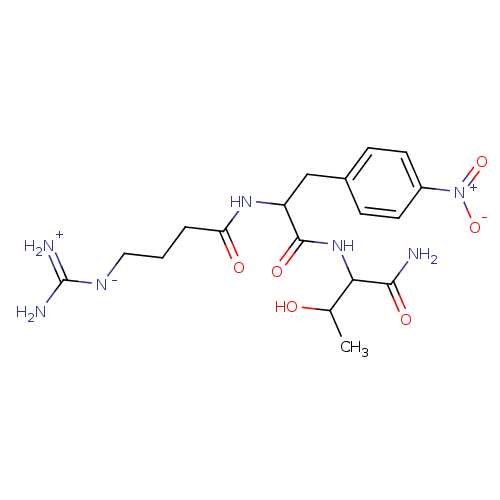 (2-[2-(4-Guanidino-butyrylamino)-3-(4-nitro-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit Human alpha-thrombin was determined | Bioorg Med Chem Lett 12: 3183-6 (2002) BindingDB Entry DOI: 10.7270/Q20P0ZBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||