

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123737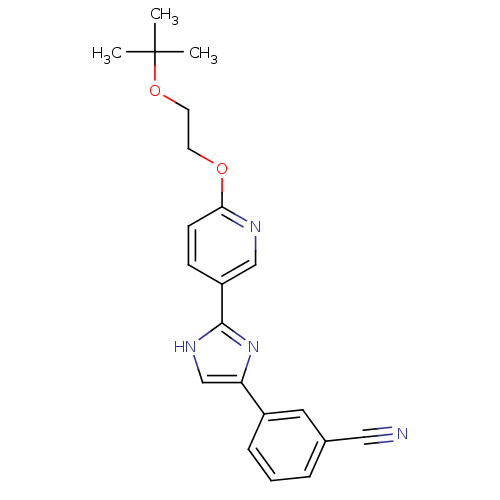 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the human Neuropeptide Y receptor type 5 was determined using [125I]- [PYY] as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50123737 (3-{2-[6-(2-tert-Butoxy-ethoxy)-pyridin-3-yl]-3H-im...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity of the compound to the rat Neuropeptide Y receptor type 5 was determined using [125I]- [Leu31,Pro34]PYY as radioligand | J Med Chem 46: 670-3 (2003) Article DOI: 10.1021/jm025584p BindingDB Entry DOI: 10.7270/Q2V987DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||