 Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50012977
Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50012977 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50092053
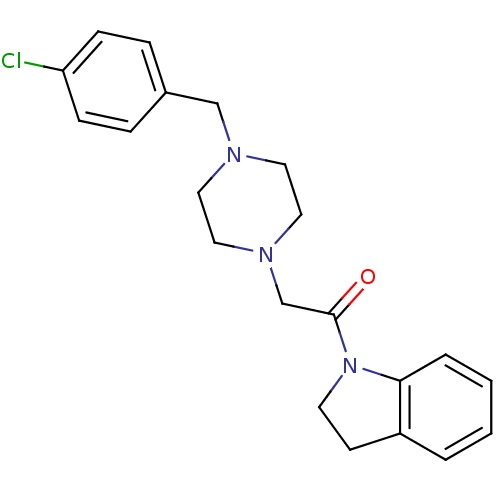
(2-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-(2,3-dihy...)Show InChI InChI=1S/C21H24ClN3O/c22-19-7-5-17(6-8-19)15-23-11-13-24(14-12-23)16-21(26)25-10-9-18-3-1-2-4-20(18)25/h1-8H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124931
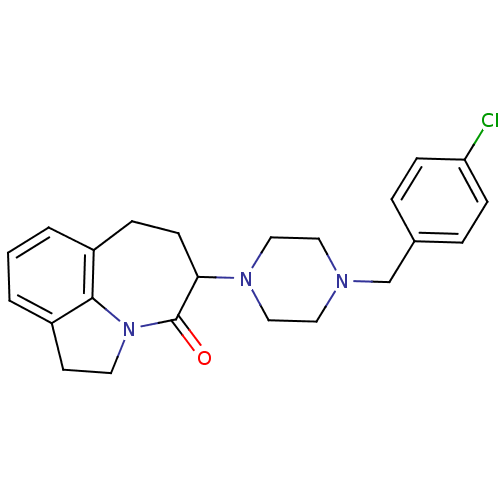
(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C23H26ClN3O/c24-20-7-4-17(5-8-20)16-25-12-14-26(15-13-25)21-9-6-18-2-1-3-19-10-11-27(22(18)19)23(21)28/h1-5,7-8,21H,6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
D4 receptor functional activity was measured inhibition of quinpirole stimulated [35S]GTP-gamma-S binding from cell membranes. |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124932

(5-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C24H29N3O/c1-18-5-7-19(8-6-18)17-25-13-15-26(16-14-25)22-10-9-20-3-2-4-21-11-12-27(23(20)21)24(22)28/h2-8,22H,9-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50092046
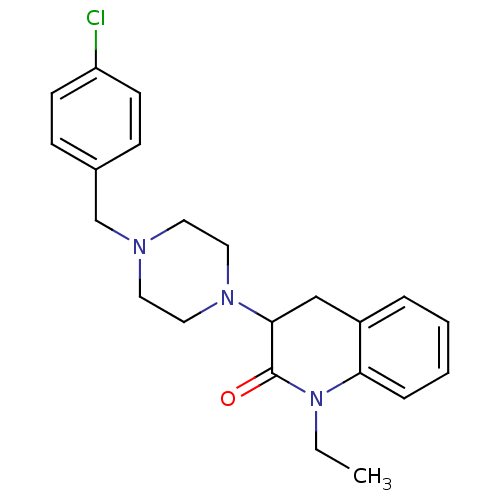
(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-3,4...)Show SMILES CCN1C(=O)C(Cc2ccccc12)N1CCN(Cc2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C22H26ClN3O/c1-2-26-20-6-4-3-5-18(20)15-21(22(26)27)25-13-11-24(12-14-25)16-17-7-9-19(23)10-8-17/h3-10,21H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124931

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C23H26ClN3O/c24-20-7-4-17(5-8-20)16-25-12-14-26(15-13-25)21-9-6-18-2-1-3-19-10-11-27(22(18)19)23(21)28/h1-5,7-8,21H,6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124934
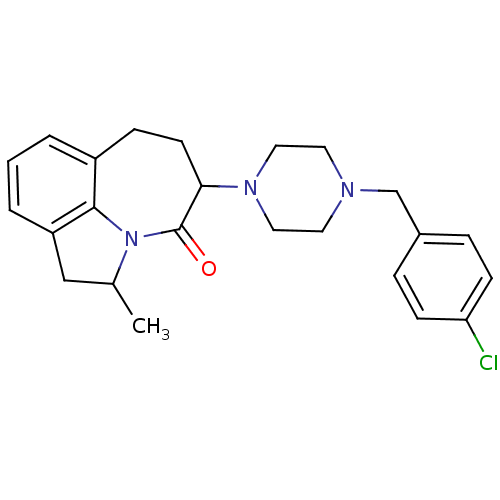
(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2-methyl-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C24H28ClN3O/c1-17-15-20-4-2-3-19-7-10-22(24(29)28(17)23(19)20)27-13-11-26(12-14-27)16-18-5-8-21(25)9-6-18/h2-6,8-9,17,22H,7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124935
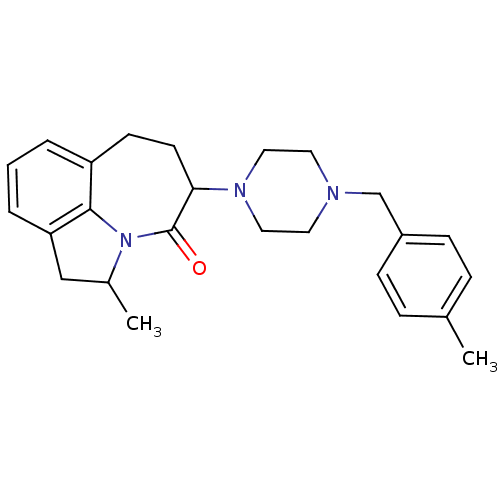
(2-Methyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl]-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(C)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H31N3O/c1-18-6-8-20(9-7-18)17-26-12-14-27(15-13-26)23-11-10-21-4-3-5-22-16-19(2)28(24(21)22)25(23)29/h3-9,19,23H,10-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124936
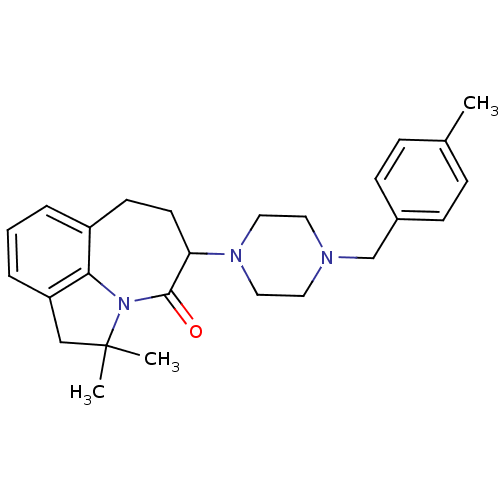
(2,2-Dimethyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CC(C)(C)N(c34)C2=O)cc1 Show InChI InChI=1S/C26H33N3O/c1-19-7-9-20(10-8-19)18-27-13-15-28(16-14-27)23-12-11-21-5-4-6-22-17-26(2,3)29(24(21)22)25(23)30/h4-10,23H,11-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124937
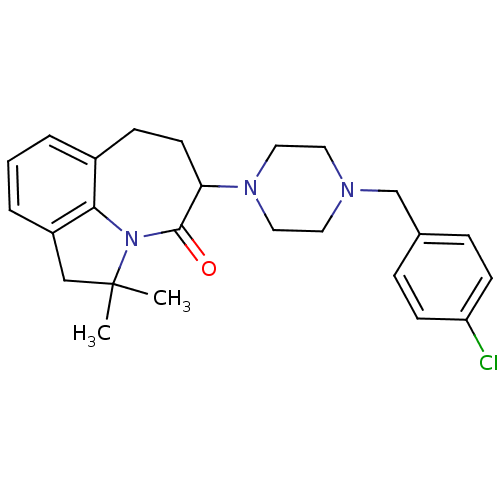
(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2,2-dimethy...)Show SMILES CC1(C)Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H30ClN3O/c1-25(2)16-20-5-3-4-19-8-11-22(24(30)29(25)23(19)20)28-14-12-27(13-15-28)17-18-6-9-21(26)10-7-18/h3-7,9-10,22H,8,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092046

(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-3,4...)Show SMILES CCN1C(=O)C(Cc2ccccc12)N1CCN(Cc2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C22H26ClN3O/c1-2-26-20-6-4-3-5-18(20)15-21(22(26)27)25-13-11-24(12-14-25)16-17-7-9-19(23)10-8-17/h3-10,21H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124935

(2-Methyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl]-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(C)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H31N3O/c1-18-6-8-20(9-7-18)17-26-12-14-27(15-13-26)23-11-10-21-4-3-5-22-16-19(2)28(24(21)22)25(23)29/h3-9,19,23H,10-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124938
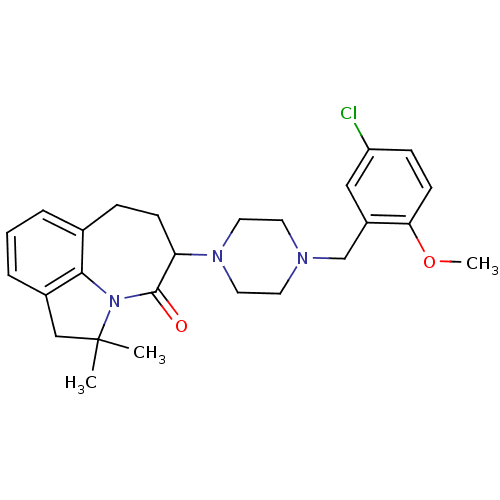
(5-[4-(5-Chloro-2-methoxy-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(Cl)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C26H32ClN3O2/c1-26(2)16-19-6-4-5-18-7-9-22(25(31)30(26)24(18)19)29-13-11-28(12-14-29)17-20-15-21(27)8-10-23(20)32-3/h4-6,8,10,15,22H,7,9,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124933
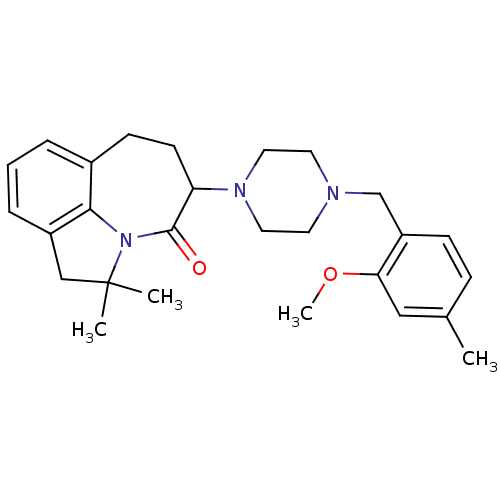
(5-[4-(2-Methoxy-4-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1cc(C)ccc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-9-22(24(16-19)32-4)18-28-12-14-29(15-13-28)23-11-10-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-9,16,23H,10-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124931

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C23H26ClN3O/c24-20-7-4-17(5-8-20)16-25-12-14-26(15-13-25)21-9-6-18-2-1-3-19-10-11-27(22(18)19)23(21)28/h1-5,7-8,21H,6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
D2 receptor functional activity was measured through reversal of quinpirole inhibited, forskolin stimulated cAMP production from whole cells |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50124939
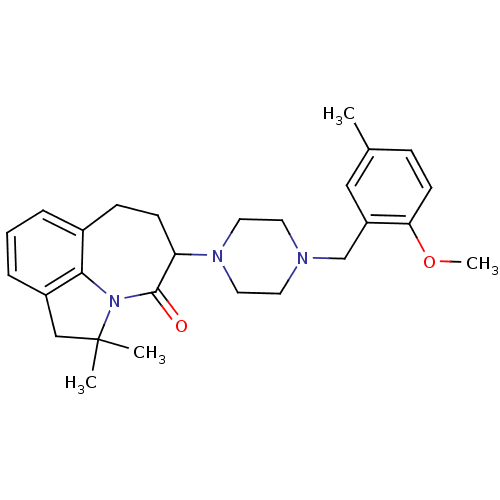
(5-[4-(2-Methoxy-5-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(C)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-11-24(32-4)22(16-19)18-28-12-14-29(15-13-28)23-10-9-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-8,11,16,23H,9-10,12-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50092053

(2-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-(2,3-dihy...)Show InChI InChI=1S/C21H24ClN3O/c22-19-7-5-17(6-8-19)15-23-11-13-24(14-12-23)16-21(26)25-10-9-18-3-1-2-4-20(18)25/h1-8H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124931

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C23H26ClN3O/c24-20-7-4-17(5-8-20)16-25-12-14-26(15-13-25)21-9-6-18-2-1-3-19-10-11-27(22(18)19)23(21)28/h1-5,7-8,21H,6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124934

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2-methyl-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C24H28ClN3O/c1-17-15-20-4-2-3-19-7-10-22(24(29)28(17)23(19)20)27-13-11-26(12-14-27)16-18-5-8-21(25)9-6-18/h2-6,8-9,17,22H,7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124936

(2,2-Dimethyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CC(C)(C)N(c34)C2=O)cc1 Show InChI InChI=1S/C26H33N3O/c1-19-7-9-20(10-8-19)18-27-13-15-28(16-14-27)23-12-11-21-5-4-6-22-17-26(2,3)29(24(21)22)25(23)30/h4-10,23H,11-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124937

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2,2-dimethy...)Show SMILES CC1(C)Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H30ClN3O/c1-25(2)16-20-5-3-4-19-8-11-22(24(30)29(25)23(19)20)28-14-12-27(13-15-28)17-18-6-9-21(26)10-7-18/h3-7,9-10,22H,8,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124932

(5-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C24H29N3O/c1-18-5-7-19(8-6-18)17-25-13-15-26(16-14-25)22-10-9-20-3-2-4-21-11-12-27(23(20)21)24(22)28/h2-8,22H,9-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 209 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124938

(5-[4-(5-Chloro-2-methoxy-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(Cl)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C26H32ClN3O2/c1-26(2)16-19-6-4-5-18-7-9-22(25(31)30(26)24(18)19)29-13-11-28(12-14-29)17-20-15-21(27)8-10-23(20)32-3/h4-6,8,10,15,22H,7,9,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124939

(5-[4-(2-Methoxy-5-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(C)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-11-24(32-4)22(16-19)18-28-12-14-29(15-13-28)23-10-9-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-8,11,16,23H,9-10,12-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124936

(2,2-Dimethyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CC(C)(C)N(c34)C2=O)cc1 Show InChI InChI=1S/C26H33N3O/c1-19-7-9-20(10-8-19)18-27-13-15-28(16-14-27)23-12-11-21-5-4-6-22-17-26(2,3)29(24(21)22)25(23)30/h4-10,23H,11-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124933

(5-[4-(2-Methoxy-4-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1cc(C)ccc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-9-22(24(16-19)32-4)18-28-12-14-29(15-13-28)23-11-10-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-9,16,23H,10-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 653 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092053

(2-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-(2,3-dihy...)Show InChI InChI=1S/C21H24ClN3O/c22-19-7-5-17(6-8-19)15-23-11-13-24(14-12-23)16-21(26)25-10-9-18-3-1-2-4-20(18)25/h1-8H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50124933

(5-[4-(2-Methoxy-4-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1cc(C)ccc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-9-22(24(16-19)32-4)18-28-12-14-29(15-13-28)23-11-10-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-9,16,23H,10-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 952 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124939

(5-[4-(2-Methoxy-5-methyl-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(C)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C27H35N3O2/c1-19-8-11-24(32-4)22(16-19)18-28-12-14-29(15-13-28)23-10-9-20-6-5-7-21-17-27(2,3)30(25(20)21)26(23)31/h5-8,11,16,23H,9-10,12-15,17-18H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 983 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124934

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2-methyl-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C24H28ClN3O/c1-17-15-20-4-2-3-19-7-10-22(24(29)28(17)23(19)20)27-13-11-26(12-14-27)16-18-5-8-21(25)9-6-18/h2-6,8-9,17,22H,7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50092054
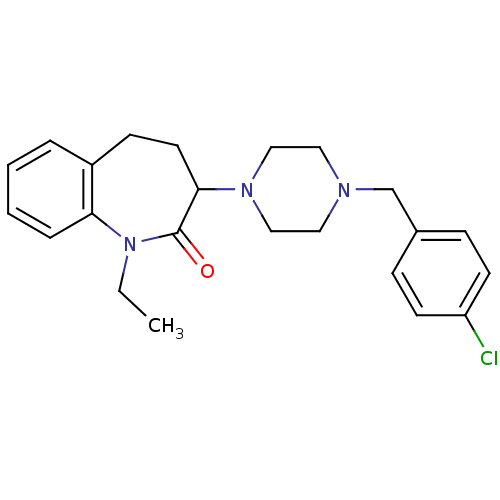
(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-1,3...)Show SMILES CCN1c2ccccc2CCC(N2CCN(Cc3ccc(Cl)cc3)CC2)C1=O Show InChI InChI=1S/C23H28ClN3O/c1-2-27-21-6-4-3-5-19(21)9-12-22(23(27)28)26-15-13-25(14-16-26)17-18-7-10-20(24)11-8-18/h3-8,10-11,22H,2,9,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124935

(2-Methyl-5-[4-(4-methyl-benzyl)-piperazin-1-yl]-1,...)Show SMILES CC1Cc2cccc3CCC(N4CCN(Cc5ccc(C)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H31N3O/c1-18-6-8-20(9-7-18)17-26-12-14-27(15-13-26)23-11-10-21-4-3-5-22-16-19(2)28(24(21)22)25(23)29/h3-9,19,23H,10-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
D4 receptor functional activity was measured inhibition of quinpirole stimulated [35S]GTP-gamma-S binding from cell membranes. |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124938

(5-[4-(5-Chloro-2-methoxy-benzyl)-piperazin-1-yl]-2...)Show SMILES COc1ccc(Cl)cc1CN1CCN(CC1)C1CCc2cccc3CC(C)(C)N(c23)C1=O Show InChI InChI=1S/C26H32ClN3O2/c1-26(2)16-19-6-4-5-18-7-9-22(25(31)30(26)24(18)19)29-13-11-28(12-14-29)17-20-15-21(27)8-10-23(20)32-3/h4-6,8,10,15,22H,7,9,11-14,16-17H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50092046

(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-3,4...)Show SMILES CCN1C(=O)C(Cc2ccccc12)N1CCN(Cc2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C22H26ClN3O/c1-2-26-20-6-4-3-5-18(20)15-21(22(26)27)25-13-11-24(12-14-25)16-17-7-9-19(23)10-8-17/h3-10,21H,2,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124932

(5-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Cc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C24H29N3O/c1-18-5-7-19(8-6-18)17-25-13-15-26(16-14-25)22-10-9-20-3-2-4-21-11-12-27(23(20)21)24(22)28/h2-8,22H,9-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50092054

(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-1,3...)Show SMILES CCN1c2ccccc2CCC(N2CCN(Cc3ccc(Cl)cc3)CC2)C1=O Show InChI InChI=1S/C23H28ClN3O/c1-2-27-21-6-4-3-5-19(21)9-12-22(23(27)28)26-15-13-25(14-16-26)17-18-7-10-20(24)11-8-18/h3-8,10-11,22H,2,9,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D4 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124937

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-2,2-dimethy...)Show SMILES CC1(C)Cc2cccc3CCC(N4CCN(Cc5ccc(Cl)cc5)CC4)C(=O)N1c23 Show InChI InChI=1S/C25H30ClN3O/c1-25(2)16-20-5-3-4-19-8-11-22(24(30)29(25)23(19)20)28-14-12-27(13-15-28)17-18-6-9-21(26)10-7-18/h3-7,9-10,22H,8,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50124931

(5-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1,2,6,7-tet...)Show SMILES Clc1ccc(CN2CCN(CC2)C2CCc3cccc4CCN(c34)C2=O)cc1 Show InChI InChI=1S/C23H26ClN3O/c24-20-7-4-17(5-8-20)16-25-12-14-26(15-13-25)21-9-6-18-2-1-3-19-10-11-27(22(18)19)23(21)28/h1-5,7-8,21H,6,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]-YM 09151 from D2 receptor |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50092054

(3-[4-(4-Chloro-benzyl)-piperazin-1-yl]-1-ethyl-1,3...)Show SMILES CCN1c2ccccc2CCC(N2CCN(Cc3ccc(Cl)cc3)CC2)C1=O Show InChI InChI=1S/C23H28ClN3O/c1-2-27-21-6-4-3-5-19(21)9-12-22(23(27)28)26-15-13-25(14-16-26)17-18-7-10-20(24)11-8-18/h3-8,10-11,22H,2,9,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha-1 adrenergic receptor of rat brain homogenate |
Bioorg Med Chem Lett 13: 701-4 (2003)
BindingDB Entry DOI: 10.7270/Q2B56J44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data