 Found 64 hits Enz. Inhib. hit(s) with all data for entry = 50013112
Found 64 hits Enz. Inhib. hit(s) with all data for entry = 50013112 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Brutons tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126739
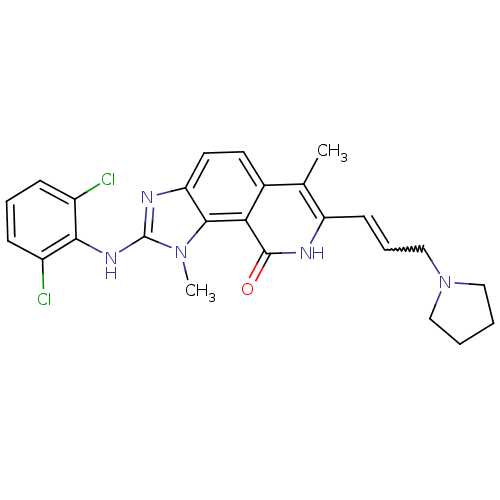
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-pyr...)Show SMILES Cc1c(C=CCN2CCCC2)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O/c1-15-16-10-11-20-23(31(2)25(29-20)30-22-17(26)7-5-8-18(22)27)21(16)24(33)28-19(15)9-6-14-32-12-3-4-13-32/h5-11H,3-4,12-14H2,1-2H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein tyrosine kinase Lyn |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126735
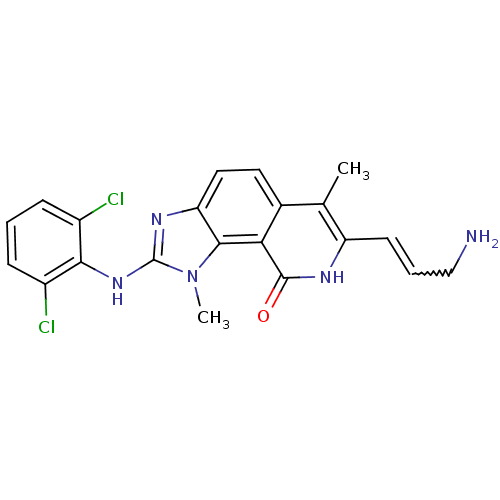
(7-(3-Amino-propenyl)-2-(2,6-dichloro-phenylamino)-...)Show SMILES Cc1c(C=CCN)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C21H19Cl2N5O/c1-11-12-8-9-16-19(17(12)20(29)25-15(11)7-4-10-24)28(2)21(26-16)27-18-13(22)5-3-6-14(18)23/h3-9H,10,24H2,1-2H3,(H,25,29)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126751
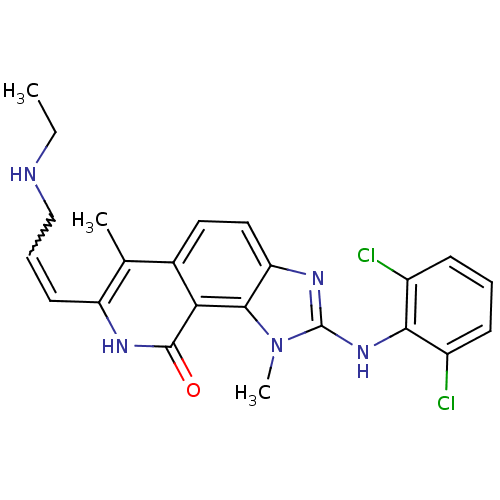
(2-(2,6-Dichloro-phenylamino)-7-(3-ethylamino-prope...)Show SMILES CCNCC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:4.3| Show InChI InChI=1S/C23H23Cl2N5O/c1-4-26-12-6-9-17-13(2)14-10-11-18-21(19(14)22(31)27-17)30(3)23(28-18)29-20-15(24)7-5-8-16(20)25/h5-11,26H,4,12H2,1-3H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126746
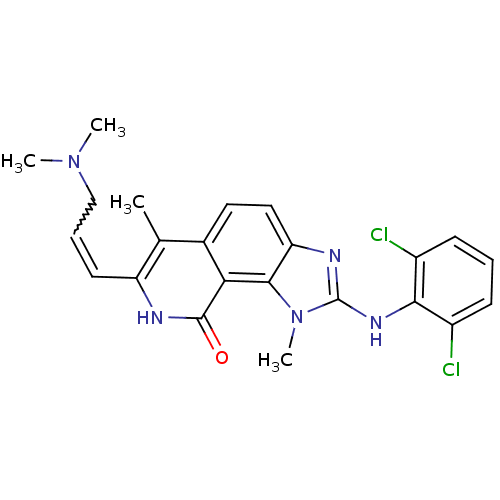
(2-(2,6-Dichloro-phenylamino)-7-(3-dimethylamino-pr...)Show SMILES CN(C)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:4.3| Show InChI InChI=1S/C23H23Cl2N5O/c1-13-14-10-11-18-21(19(14)22(31)26-17(13)9-6-12-29(2)3)30(4)23(27-18)28-20-15(24)7-5-8-16(20)25/h5-11H,12H2,1-4H3,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126749
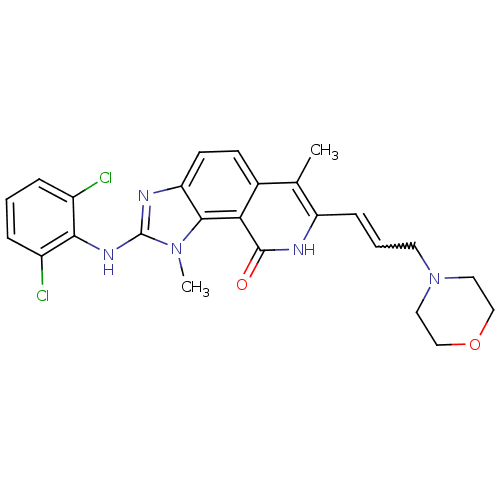
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-mor...)Show SMILES Cc1c(C=CCN2CCOCC2)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O2/c1-15-16-8-9-20-23(31(2)25(29-20)30-22-17(26)5-3-6-18(22)27)21(16)24(33)28-19(15)7-4-10-32-11-13-34-14-12-32/h3-9H,10-14H2,1-2H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126752
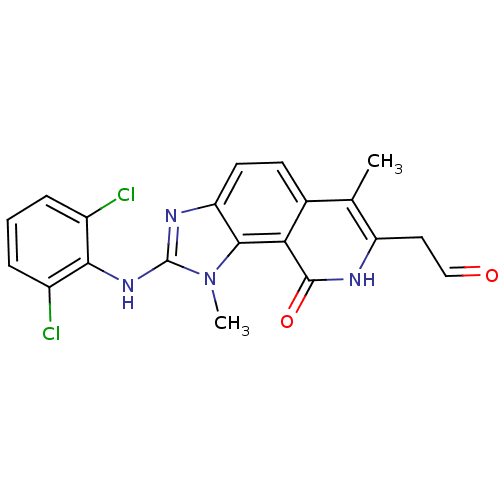
(2-(2,6-Dichloro-phenylamino)-7-(2-hydroxy-vinyl)-1...)Show SMILES Cc1c(CC=O)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-10-11-6-7-15-18(16(11)19(28)23-14(10)8-9-27)26(2)20(24-15)25-17-12(21)4-3-5-13(17)22/h3-7,9H,8H2,1-2H3,(H,23,28)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126743
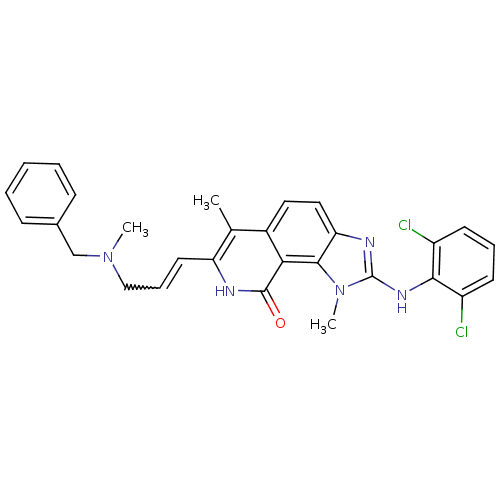
(7-[3-(Benzyl-methyl-amino)-propenyl]-2-(2,6-dichlo...)Show SMILES CN(CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C)Cc1ccccc1 |w:3.2| Show InChI InChI=1S/C29H27Cl2N5O/c1-18-20-14-15-24-27(36(3)29(33-24)34-26-21(30)11-7-12-22(26)31)25(20)28(37)32-23(18)13-8-16-35(2)17-19-9-5-4-6-10-19/h4-15H,16-17H2,1-3H3,(H,32,37)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126734
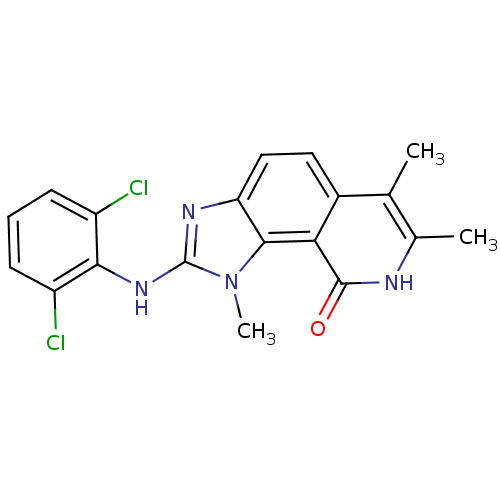
(2-(2,6-Dichloro-phenylamino)-1,6,7-trimethyl-1,8-d...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C Show InChI InChI=1S/C19H16Cl2N4O/c1-9-10(2)22-18(26)15-11(9)7-8-14-17(15)25(3)19(23-14)24-16-12(20)5-4-6-13(16)21/h4-8H,1-3H3,(H,22,26)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126736
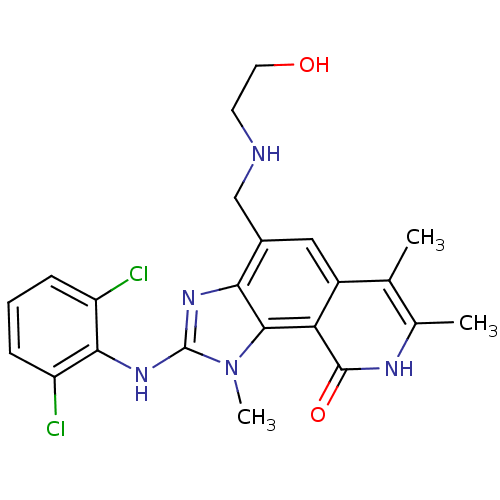
(2-(2,6-Dichloro-phenylamino)-4-[(2-hydroxy-ethylam...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3c(CNCCO)cc2c1C Show InChI InChI=1S/C22H23Cl2N5O2/c1-11-12(2)26-21(31)17-14(11)9-13(10-25-7-8-30)18-20(17)29(3)22(27-18)28-19-15(23)5-4-6-16(19)24/h4-6,9,25,30H,7-8,10H2,1-3H3,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126730
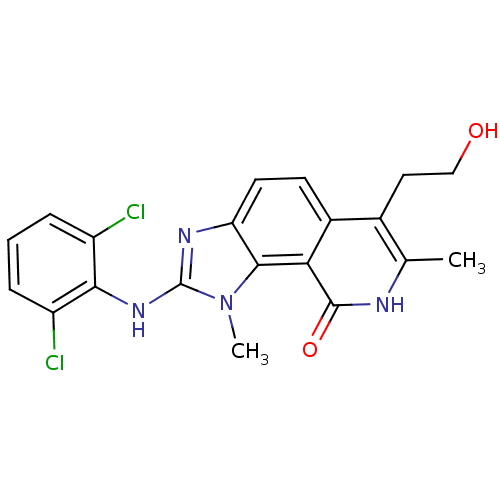
(2-(2,6-Dichloro-phenylamino)-6-(2-hydroxy-ethyl)-1...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CCO Show InChI InChI=1S/C20H18Cl2N4O2/c1-10-11(8-9-27)12-6-7-15-18(16(12)19(28)23-10)26(2)20(24-15)25-17-13(21)4-3-5-14(17)22/h3-7,27H,8-9H2,1-2H3,(H,23,28)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126738
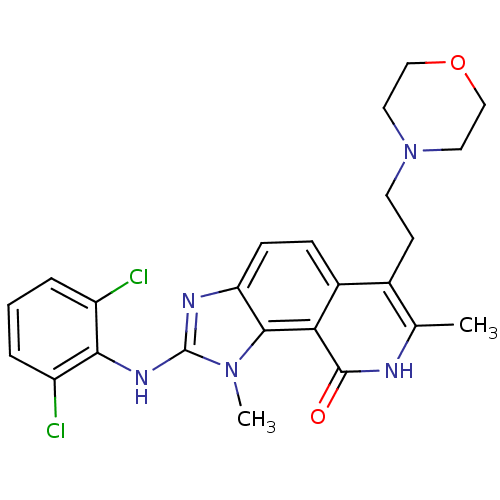
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(2-mor...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CCN1CCOCC1 Show InChI InChI=1S/C24H25Cl2N5O2/c1-14-15(8-9-31-10-12-33-13-11-31)16-6-7-19-22(20(16)23(32)27-14)30(2)24(28-19)29-21-17(25)4-3-5-18(21)26/h3-7H,8-13H2,1-2H3,(H,27,32)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126748
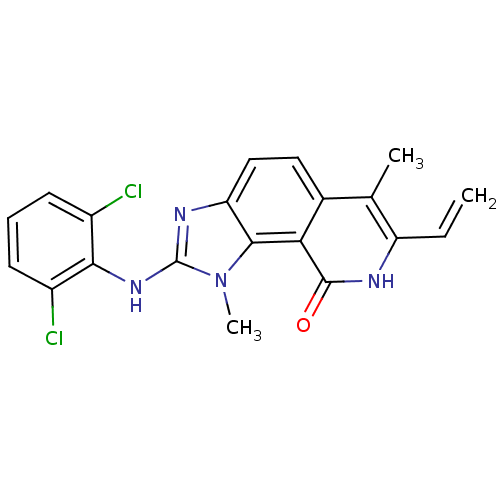
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-vinyl-...)Show SMILES Cc1c(C=C)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C20H16Cl2N4O/c1-4-14-10(2)11-8-9-15-18(16(11)19(27)23-14)26(3)20(24-15)25-17-12(21)6-5-7-13(17)22/h4-9H,1H2,2-3H3,(H,23,27)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126756
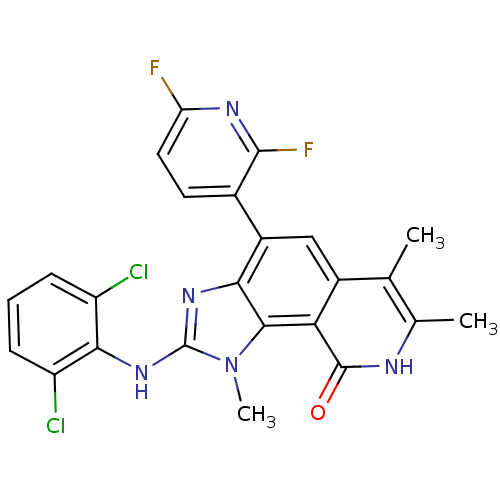
(2-(2,6-Dichloro-phenylamino)-4-(2,6-difluoro-pyrid...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3c(cc2c1C)-c1ccc(F)nc1F Show InChI InChI=1S/C24H17Cl2F2N5O/c1-10-11(2)29-23(34)18-13(10)9-14(12-7-8-17(27)30-22(12)28)19-21(18)33(3)24(31-19)32-20-15(25)5-4-6-16(20)26/h4-9H,1-3H3,(H,29,34)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126731
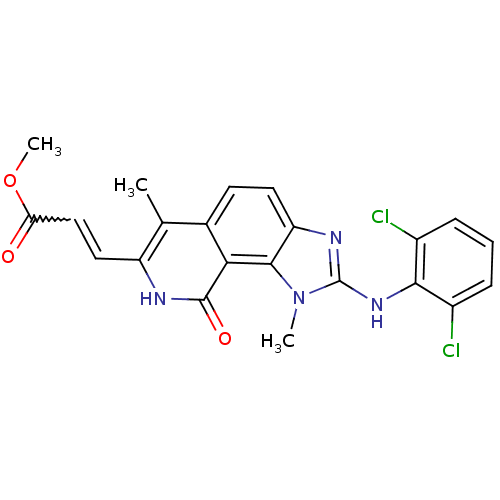
(3-[2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-9-oxo...)Show SMILES COC(=O)C=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:4.3| Show InChI InChI=1S/C22H18Cl2N4O3/c1-11-12-7-8-16-20(18(12)21(30)25-15(11)9-10-17(29)31-3)28(2)22(26-16)27-19-13(23)5-4-6-14(19)24/h4-10H,1-3H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126759
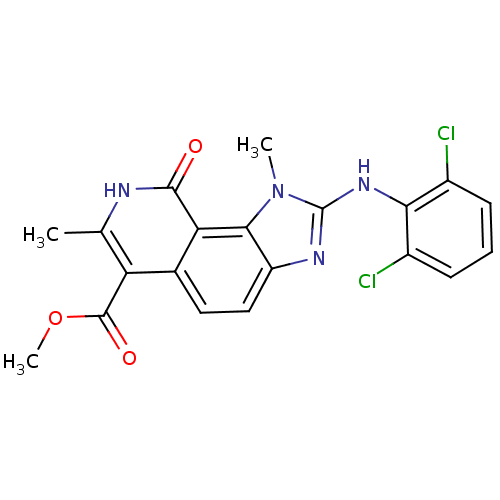
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-9-oxo-8,...)Show SMILES COC(=O)c1c(C)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C20H16Cl2N4O3/c1-9-14(19(28)29-3)10-7-8-13-17(15(10)18(27)23-9)26(2)20(24-13)25-16-11(21)5-4-6-12(16)22/h4-8H,1-3H3,(H,23,27)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50116391
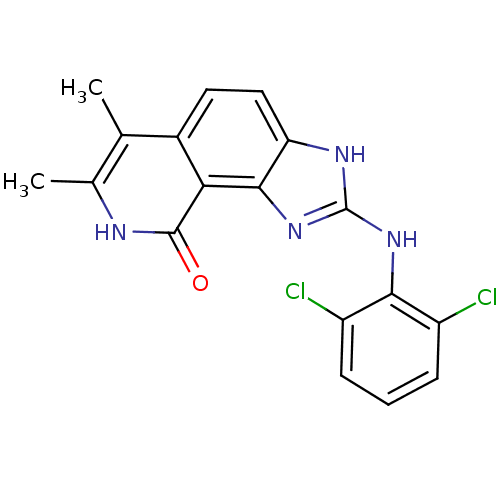
(2-(2,6-Dichloro-phenylamino)-6,7-dimethyl-1,8-dihy...)Show SMILES Cc1[nH]c(=O)c2c3nc(Nc4c(Cl)cccc4Cl)[nH]c3ccc2c1C Show InChI InChI=1S/C18H14Cl2N4O/c1-8-9(2)21-17(25)14-10(8)6-7-13-16(14)24-18(22-13)23-15-11(19)4-3-5-12(15)20/h3-7H,1-2H3,(H,21,25)(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126753
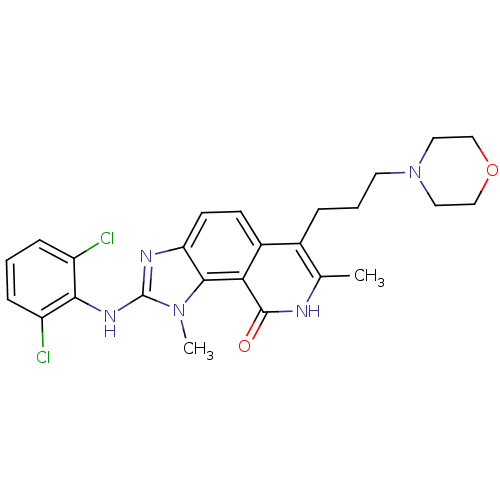
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(3-mor...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CCCN1CCOCC1 Show InChI InChI=1S/C25H27Cl2N5O2/c1-15-16(5-4-10-32-11-13-34-14-12-32)17-8-9-20-23(21(17)24(33)28-15)31(2)25(29-20)30-22-18(26)6-3-7-19(22)27/h3,6-9H,4-5,10-14H2,1-2H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126747
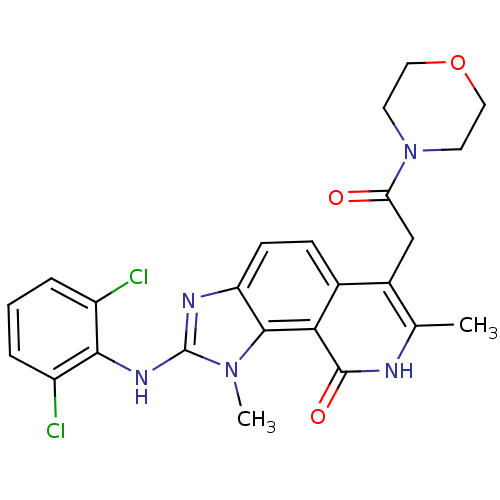
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(2-mor...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CC(=O)N1CCOCC1 Show InChI InChI=1S/C24H23Cl2N5O3/c1-13-15(12-19(32)31-8-10-34-11-9-31)14-6-7-18-22(20(14)23(33)27-13)30(2)24(28-18)29-21-16(25)4-3-5-17(21)26/h3-7H,8-12H2,1-2H3,(H,27,33)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126760
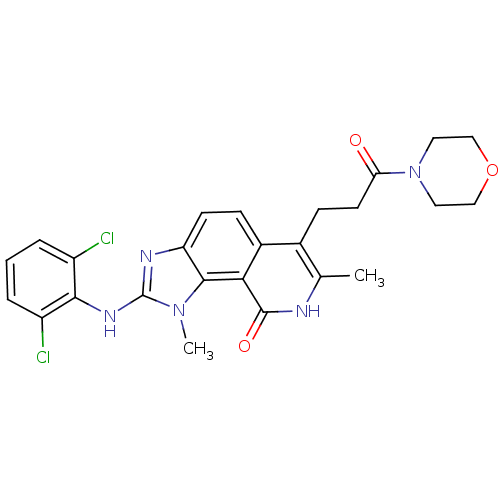
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(3-mor...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CCC(=O)N1CCOCC1 Show InChI InChI=1S/C25H25Cl2N5O3/c1-14-15(7-9-20(33)32-10-12-35-13-11-32)16-6-8-19-23(21(16)24(34)28-14)31(2)25(29-19)30-22-17(26)4-3-5-18(22)27/h3-6,8H,7,9-13H2,1-2H3,(H,28,34)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126755
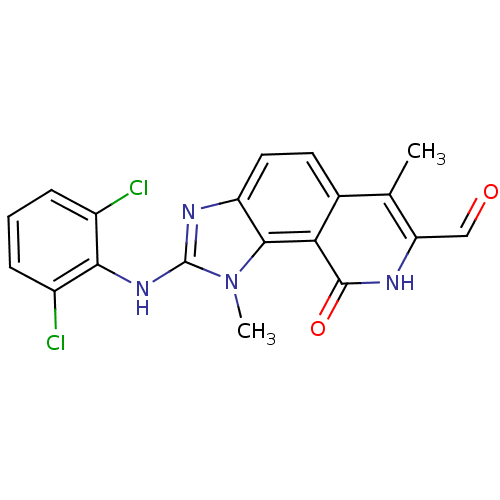
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-9-oxo-8,...)Show SMILES Cc1c(C=O)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C19H14Cl2N4O2/c1-9-10-6-7-13-17(15(10)18(27)22-14(9)8-26)25(2)19(23-13)24-16-11(20)4-3-5-12(16)21/h3-8H,1-2H3,(H,22,27)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126744
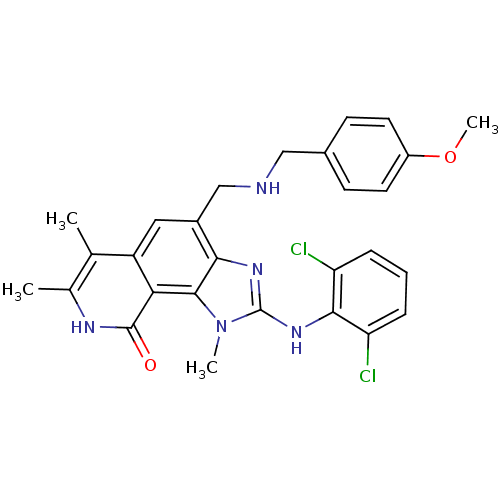
(2-(2,6-Dichloro-phenylamino)-4-[(4-methoxy-benzyla...)Show SMILES COc1ccc(CNCc2cc3c(C)c(C)[nH]c(=O)c3c3n(C)c(Nc4c(Cl)cccc4Cl)nc23)cc1 Show InChI InChI=1S/C28H27Cl2N5O2/c1-15-16(2)32-27(36)23-20(15)12-18(14-31-13-17-8-10-19(37-4)11-9-17)24-26(23)35(3)28(33-24)34-25-21(29)6-5-7-22(25)30/h5-12,31H,13-14H2,1-4H3,(H,32,36)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126729
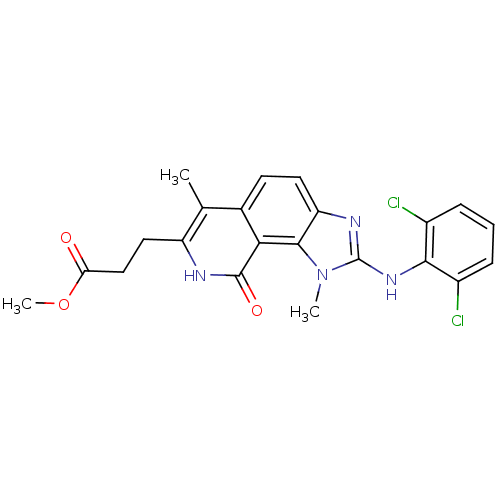
(3-[2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-9-oxo...)Show SMILES COC(=O)CCc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C Show InChI InChI=1S/C22H20Cl2N4O3/c1-11-12-7-8-16-20(18(12)21(30)25-15(11)9-10-17(29)31-3)28(2)22(26-16)27-19-13(23)5-4-6-14(19)24/h4-8H,9-10H2,1-3H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP-70) |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126742
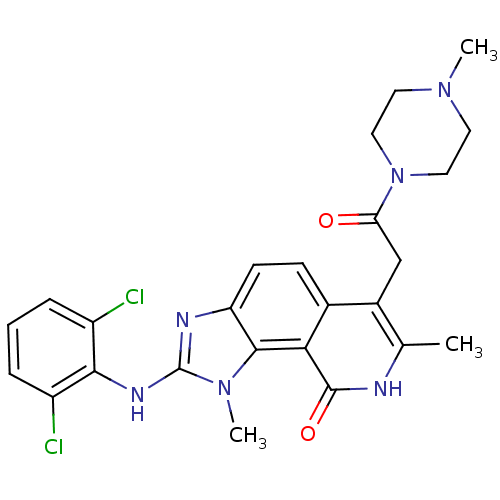
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-[2-(4-...)Show SMILES CN1CCN(CC1)C(=O)Cc1c(C)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C25H26Cl2N6O2/c1-14-16(13-20(34)33-11-9-31(2)10-12-33)15-7-8-19-23(21(15)24(35)28-14)32(3)25(29-19)30-22-17(26)5-4-6-18(22)27/h4-8H,9-13H2,1-3H3,(H,28,35)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126740
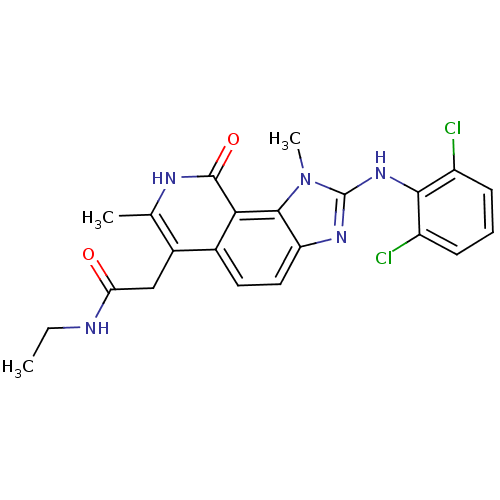
(2-[2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-9-oxo...)Show SMILES CCNC(=O)Cc1c(C)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C22H21Cl2N5O2/c1-4-25-17(30)10-13-11(2)26-21(31)18-12(13)8-9-16-20(18)29(3)22(27-16)28-19-14(23)6-5-7-15(19)24/h5-9H,4,10H2,1-3H3,(H,25,30)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126754
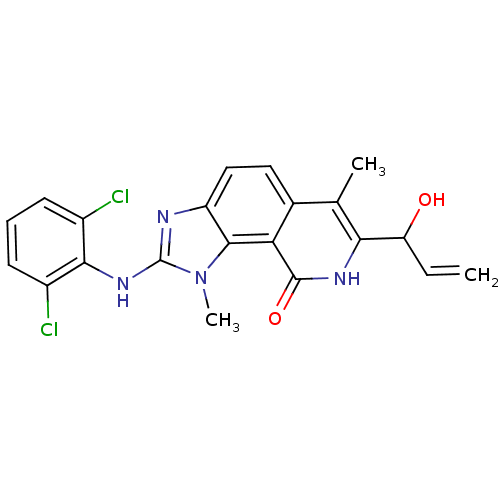
(2-(2,6-Dichloro-phenylamino)-7-(1-hydroxy-allyl)-1...)Show SMILES Cc1c([nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12)C(O)C=C Show InChI InChI=1S/C21H18Cl2N4O2/c1-4-15(28)17-10(2)11-8-9-14-19(16(11)20(29)25-17)27(3)21(24-14)26-18-12(22)6-5-7-13(18)23/h4-9,15,28H,1H2,2-3H3,(H,24,26)(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126741
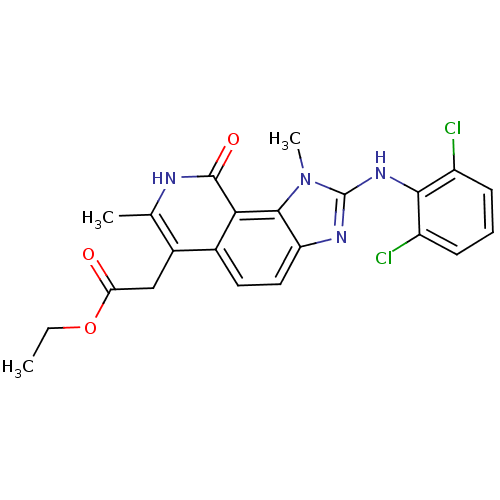
(CHEMBL281271 | [2-(2,6-Dichloro-phenylamino)-1,7-d...)Show SMILES CCOC(=O)Cc1c(C)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C22H20Cl2N4O3/c1-4-31-17(29)10-13-11(2)25-21(30)18-12(13)8-9-16-20(18)28(3)22(26-16)27-19-14(23)6-5-7-15(19)24/h5-9H,4,10H2,1-3H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126745
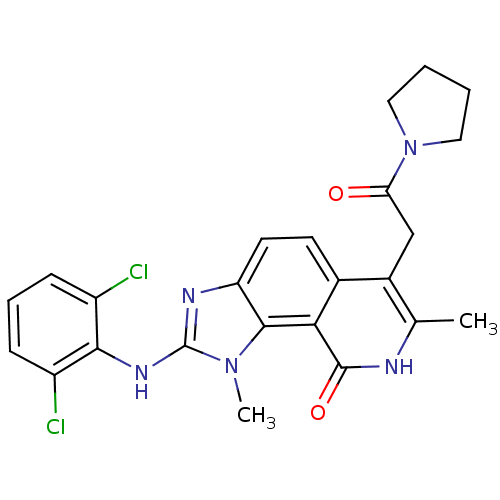
(2-(2,6-Dichloro-phenylamino)-1,7-dimethyl-6-(2-oxo...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1CC(=O)N1CCCC1 Show InChI InChI=1S/C24H23Cl2N5O2/c1-13-15(12-19(32)31-10-3-4-11-31)14-8-9-18-22(20(14)23(33)27-13)30(2)24(28-18)29-21-16(25)6-5-7-17(21)26/h5-9H,3-4,10-12H2,1-2H3,(H,27,33)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126733
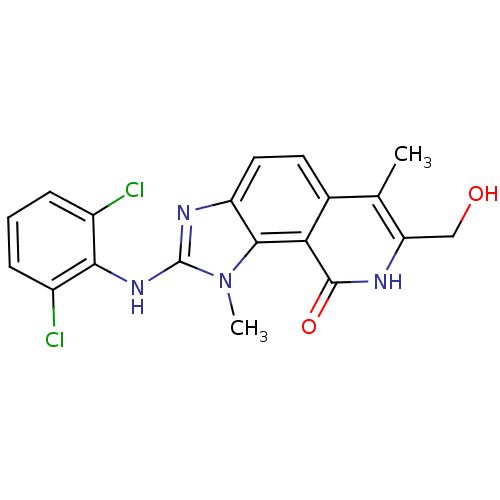
(2-(2,6-Dichloro-phenylamino)-7-hydroxymethyl-1,6-d...)Show SMILES Cc1c(CO)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C19H16Cl2N4O2/c1-9-10-6-7-13-17(15(10)18(27)22-14(9)8-26)25(2)19(23-13)24-16-11(20)4-3-5-12(16)21/h3-7,26H,8H2,1-2H3,(H,22,27)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126757
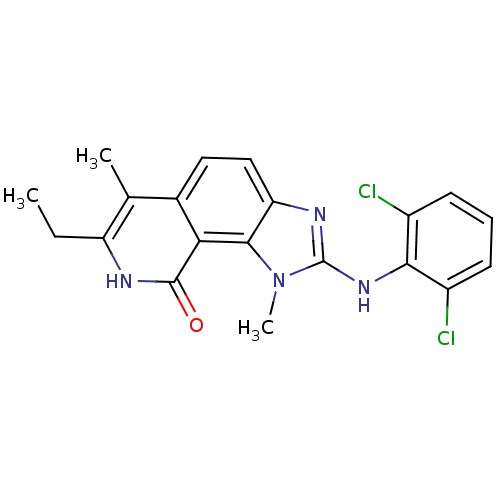
(2-(2,6-Dichloro-phenylamino)-7-ethyl-1,6-dimethyl-...)Show SMILES CCc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C Show InChI InChI=1S/C20H18Cl2N4O/c1-4-14-10(2)11-8-9-15-18(16(11)19(27)23-14)26(3)20(24-15)25-17-12(21)6-5-7-13(17)22/h5-9H,4H2,1-3H3,(H,23,27)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MSK-1 kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Serum/glucocorticoid regulated kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MAP kinase-activated protein kinase 1b/MAP kinase-activated protein kinase 2 |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10/8/9
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine-protein kinase Chk1 |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase ROCK2 |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1/beta-2
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Ribosomal protein S6 kinase (P70S6K kinase) |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase PRAK |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Phospho-inositide dependent kinase 1 (PDK1) |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase B alpha |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126758

(2-(2,6-Dichloro-phenylamino)-3,6,7-trimethyl-3,8-d...)Show SMILES Cc1[nH]c(=O)c2c3nc(Nc4c(Cl)cccc4Cl)n(C)c3ccc2c1C Show InChI InChI=1S/C19H16Cl2N4O/c1-9-10(2)22-18(26)15-11(9)7-8-14-17(15)24-19(25(14)3)23-16-12(20)5-4-6-13(16)21/h4-8H,1-3H3,(H,22,26)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126737
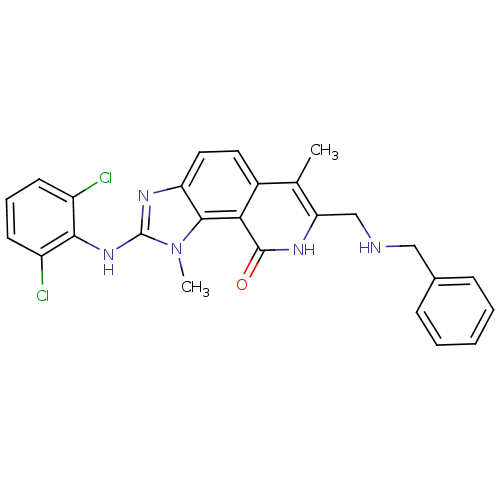
(7-(Benzylamino-methyl)-2-(2,6-dichloro-phenylamino...)Show SMILES Cc1c(CNCc2ccccc2)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C26H23Cl2N5O/c1-15-17-11-12-20-24(33(2)26(31-20)32-23-18(27)9-6-10-19(23)28)22(17)25(34)30-21(15)14-29-13-16-7-4-3-5-8-16/h3-12,29H,13-14H2,1-2H3,(H,30,34)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data