

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969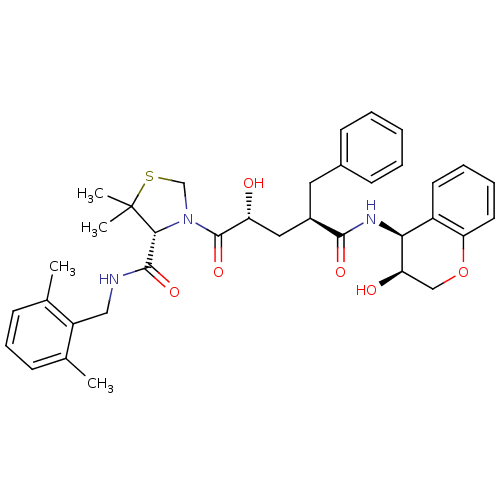 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970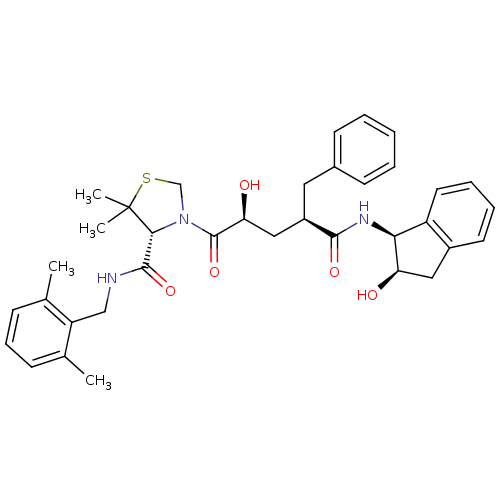 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127977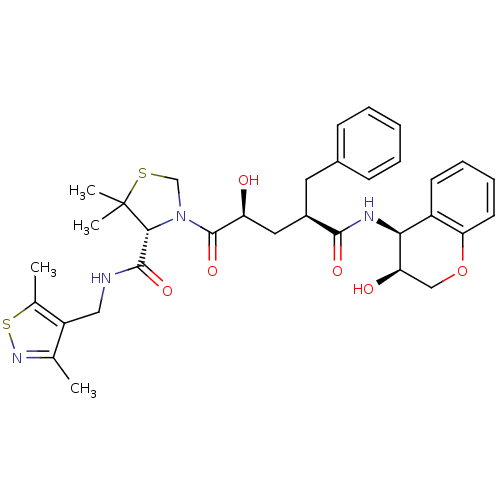 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127970 (3-[(1R,4S)-2-Hydroxy-4-((1S,2R)-2-hydroxy-indan-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127968 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127972 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127969 ((R)-3-[(2R,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127977 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127966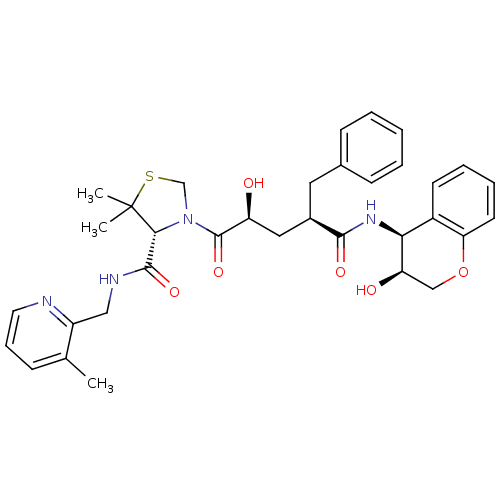 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127973 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127972 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127976 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127967 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127977 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127966 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127975 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127968 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127966 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127973 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127968 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127972 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127975 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127967 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127975 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127967 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127973 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127971 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127971 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV K-60C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127971 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV protease of HIV V-18C mutant strain | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||