

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138607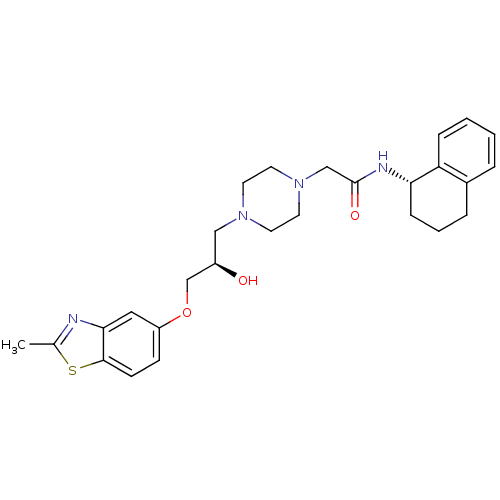 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138628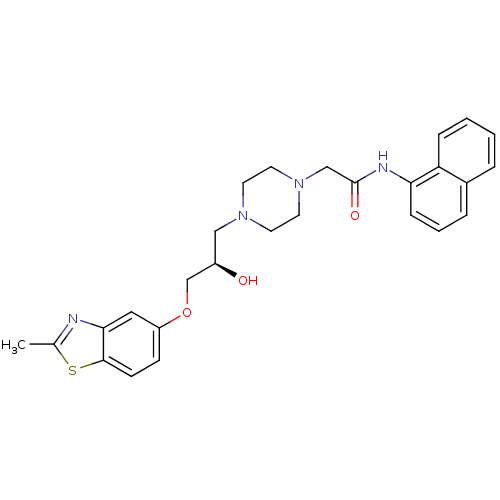 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138619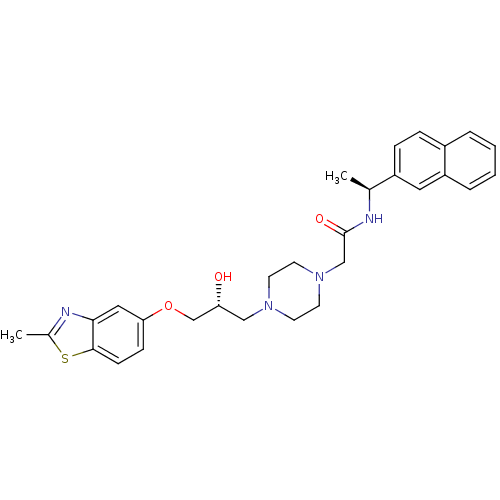 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138602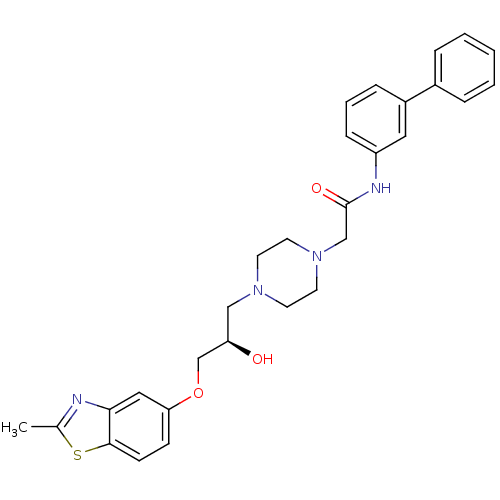 (CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138617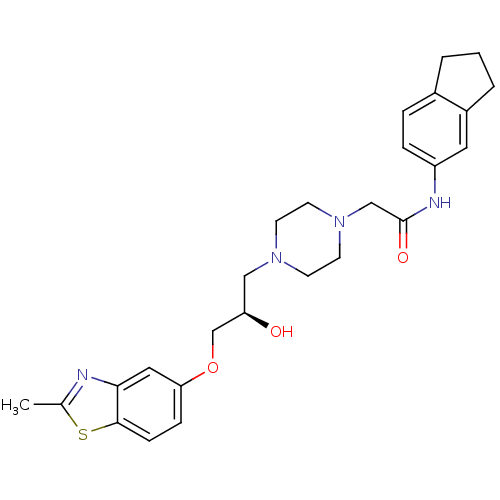 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138625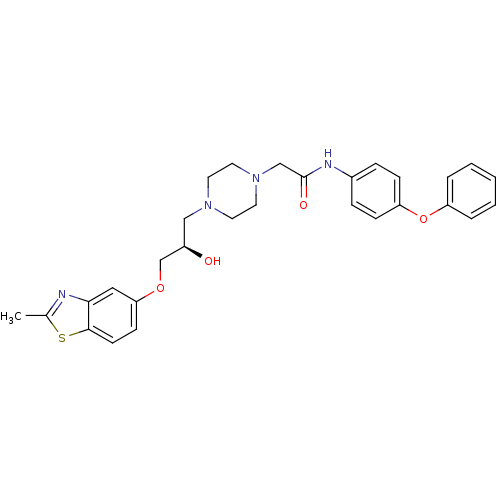 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138627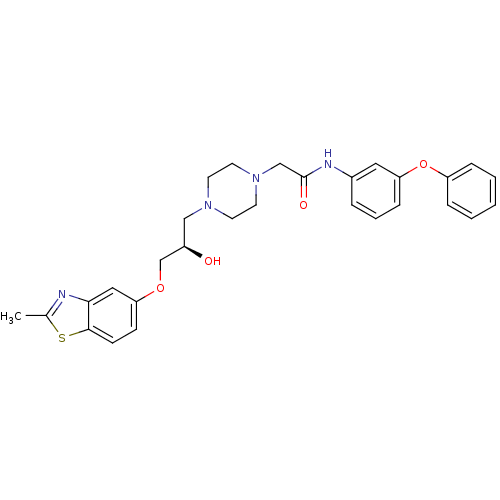 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138598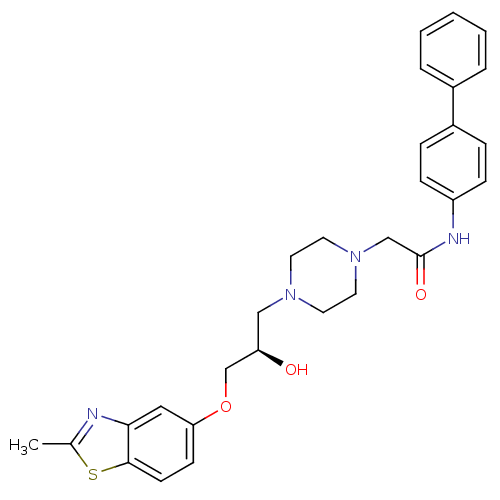 (CHEMBL153741 | N-Biphenyl-4-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138629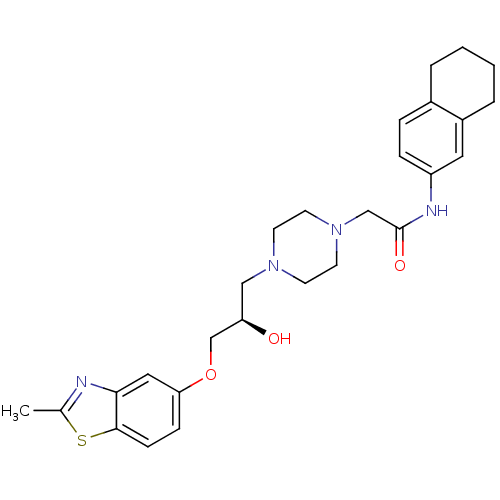 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138609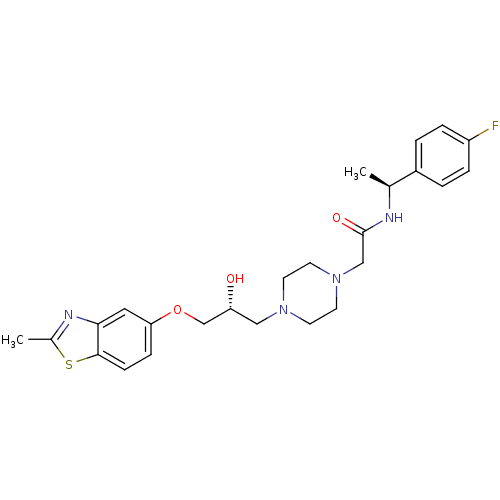 (CHEMBL155284 | N-[1-((S)-4-Fluoro-phenyl)-ethyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138611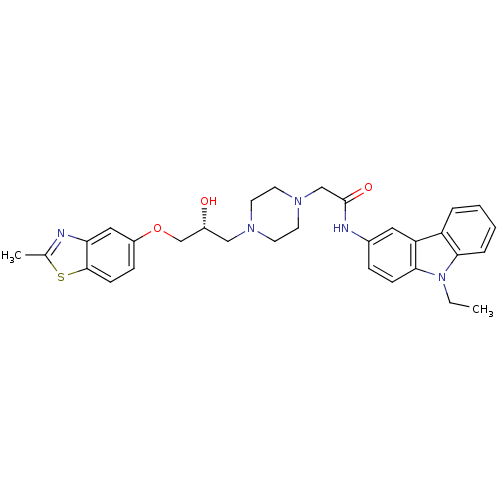 (CHEMBL150614 | N-(9-Ethyl-9H-carbazol-3-yl)-2-{4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138606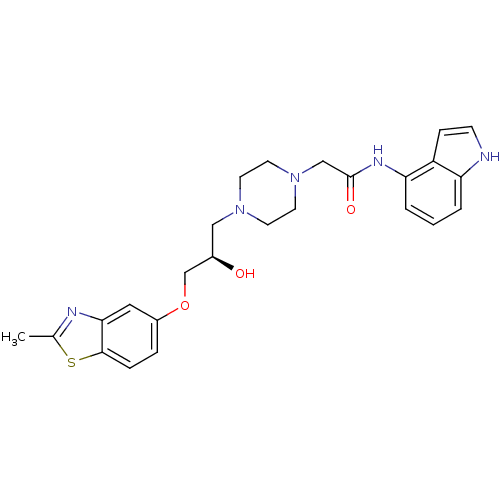 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138613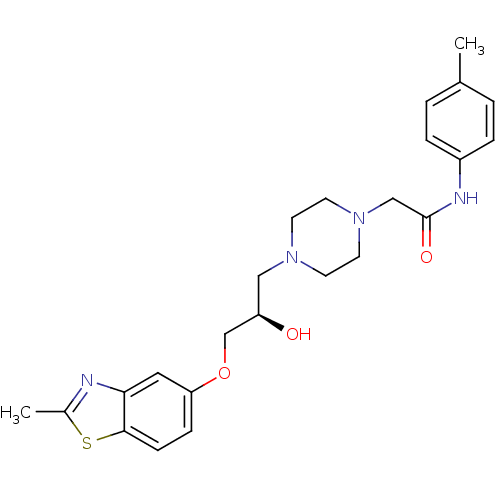 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138626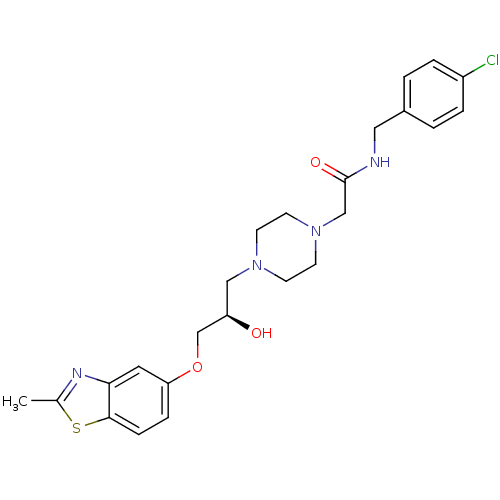 (CHEMBL152947 | N-(4-Chloro-benzyl)-2-{4-[(R)-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138618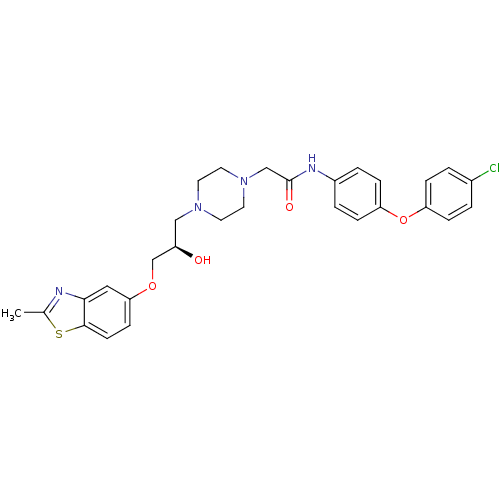 (CHEMBL152844 | N-[4-(4-Chloro-phenoxy)-phenyl]-2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138630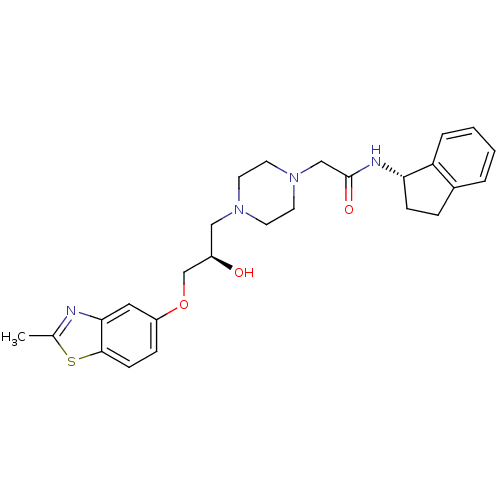 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138599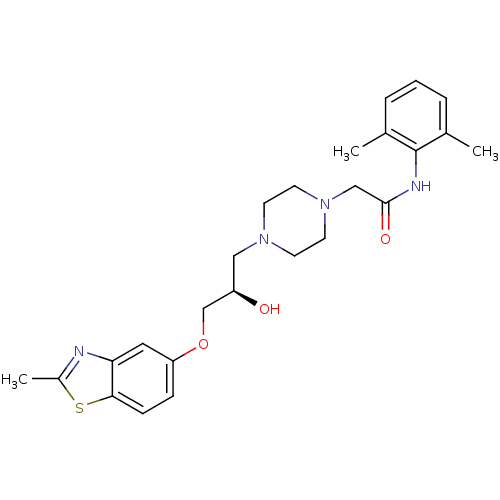 (CHEMBL154751 | N-(2,6-Dimethyl-phenyl)-2-{4-[(R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138604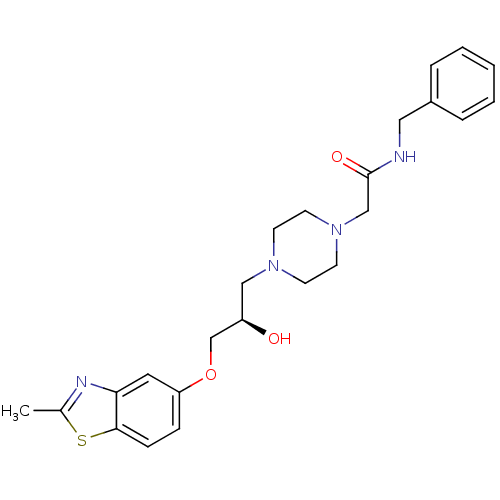 (CHEMBL153159 | N-Benzyl-2-{4-[(R)-2-hydroxy-3-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138623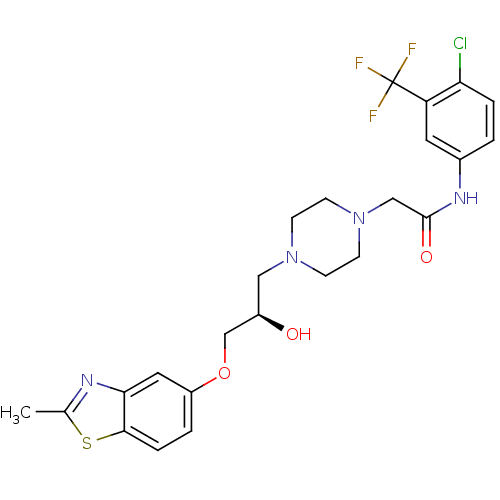 (CHEMBL357278 | N-(4-Chloro-3-trifluoromethyl-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138621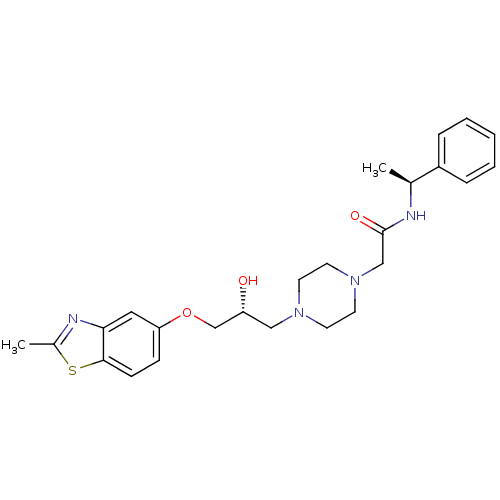 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138610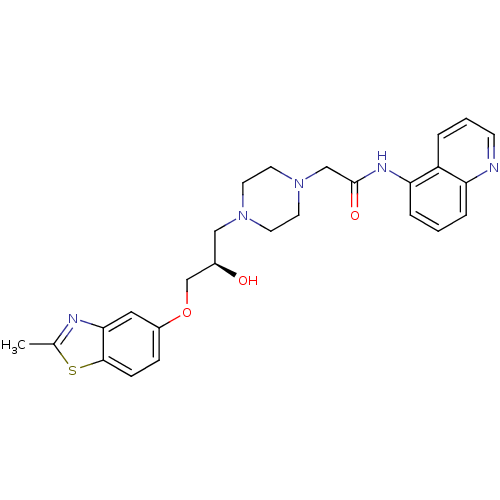 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138615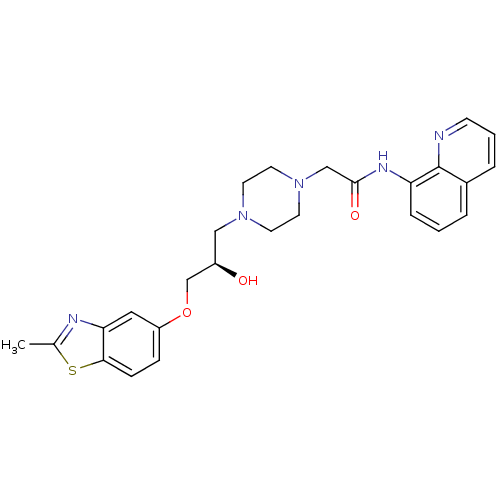 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138601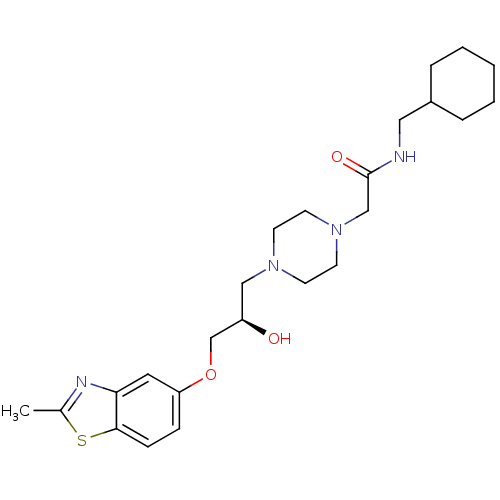 (CHEMBL153122 | N-Cyclohexylmethyl-2-{4-[(R)-2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138600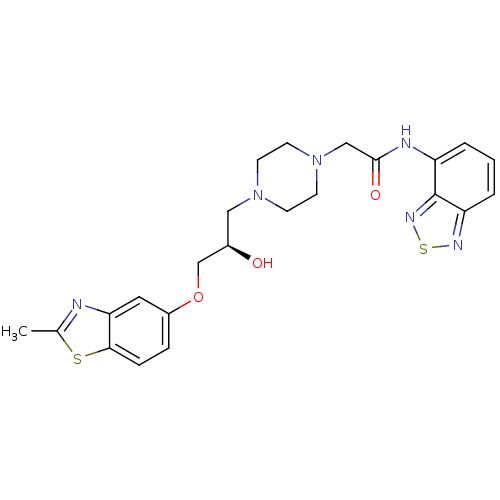 (CHEMBL152941 | N-Benzo[1,2,5]thiadiazol-4-yl-2-{4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50138602 (CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 1A2 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138631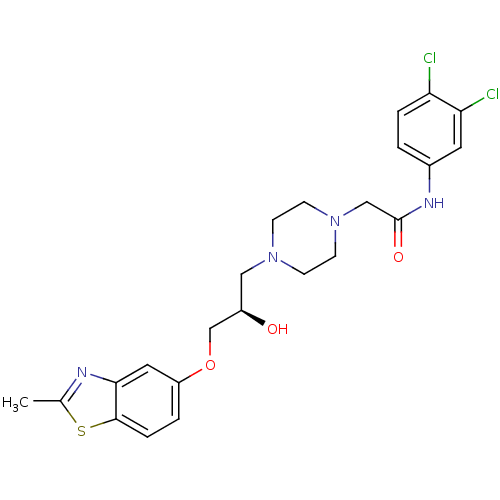 (CHEMBL153650 | N-(3,4-Dichloro-phenyl)-2-{4-[(R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138622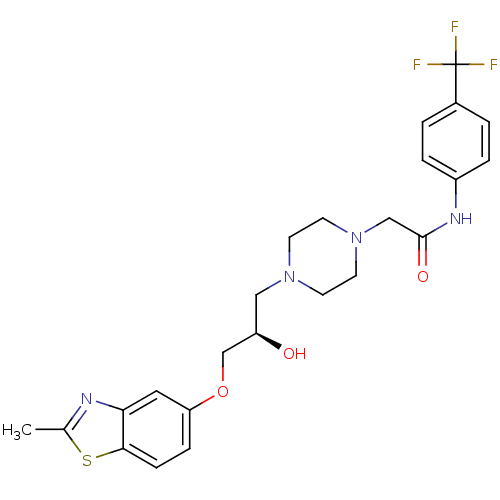 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50138598 (CHEMBL153741 | N-Biphenyl-4-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 3A4 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138614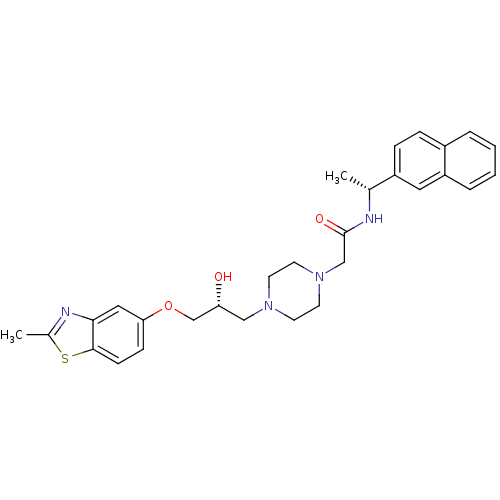 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138612 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138620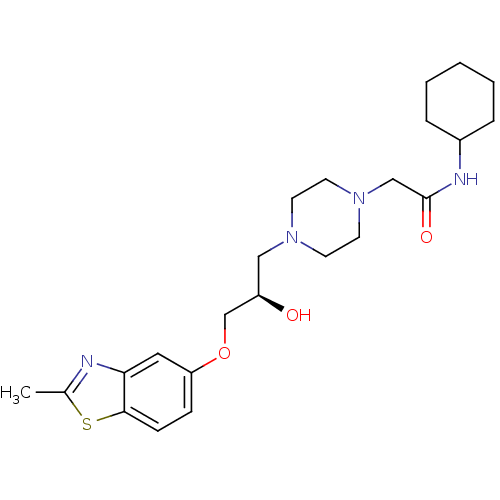 (CHEMBL155166 | N-Cyclohexyl-2-{4-[(R)-2-hydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138624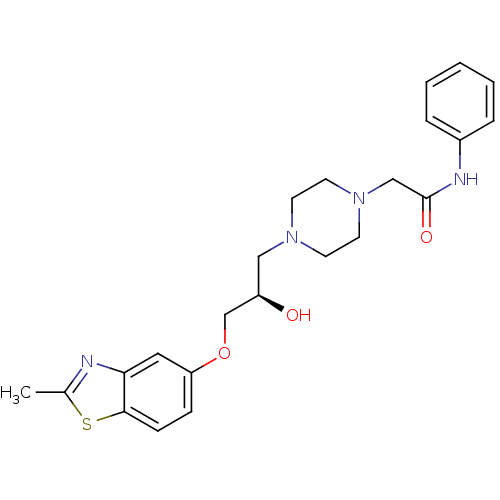 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138616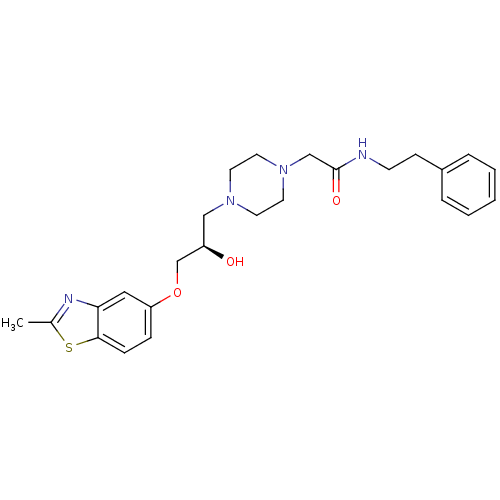 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138608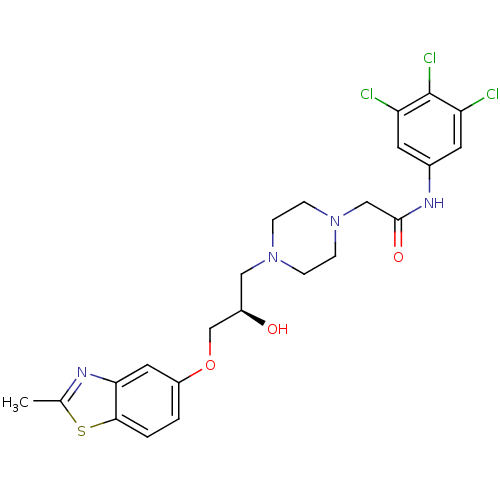 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50138599 (CHEMBL154751 | N-(2,6-Dimethyl-phenyl)-2-{4-[(R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 3A4 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50138602 (CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2C9 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138605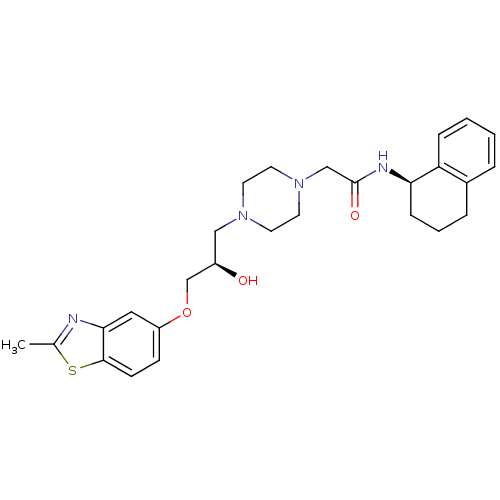 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138597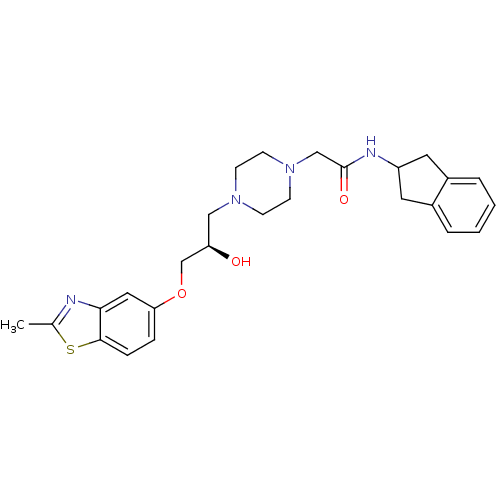 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50138598 (CHEMBL153741 | N-Biphenyl-4-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2C9 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50138598 (CHEMBL153741 | N-Biphenyl-4-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 1A2 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138603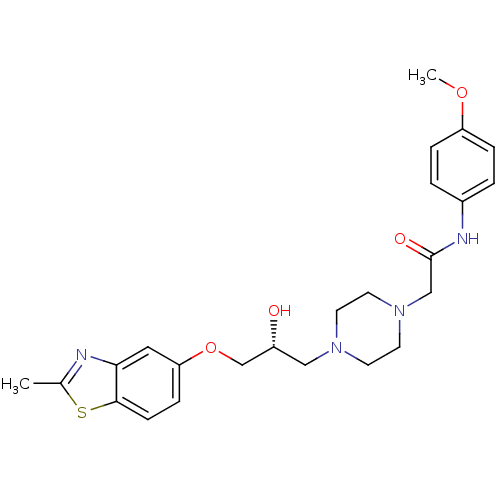 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50138602 (CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 3A4 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50138611 (CHEMBL150614 | N-(9-Ethyl-9H-carbazol-3-yl)-2-{4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 3A4 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50138611 (CHEMBL150614 | N-(9-Ethyl-9H-carbazol-3-yl)-2-{4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 1A2 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50138599 (CHEMBL154751 | N-(2,6-Dimethyl-phenyl)-2-{4-[(R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 1A2 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50138599 (CHEMBL154751 | N-(2,6-Dimethyl-phenyl)-2-{4-[(R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2C9 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50138611 (CHEMBL150614 | N-(9-Ethyl-9H-carbazol-3-yl)-2-{4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of cytochrome P450 2C9 of isolated guinea pig heart | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||