

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50326055 ((1R,21S,24S)-21-tert-Butyl-N-((1R,2R)-1-{[(cyclopr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50142916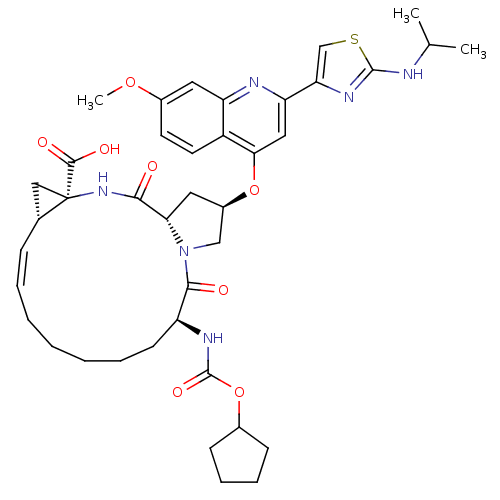 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336545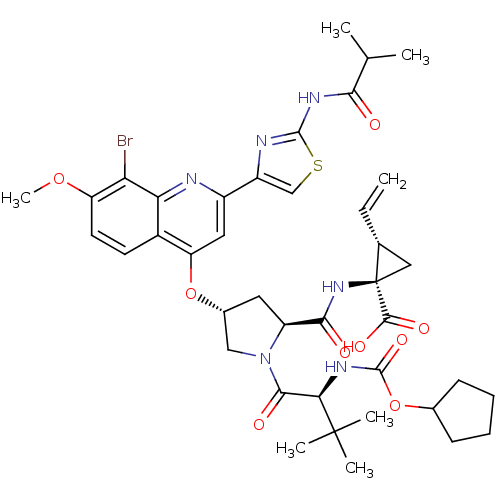 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336545 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b NS3/4A protease | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM12311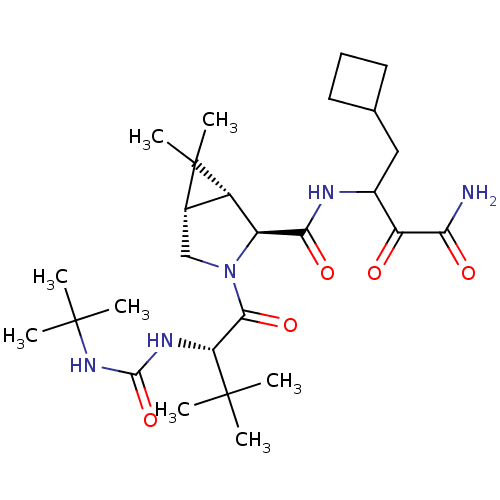 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037468 (CHEMBL3125046) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037467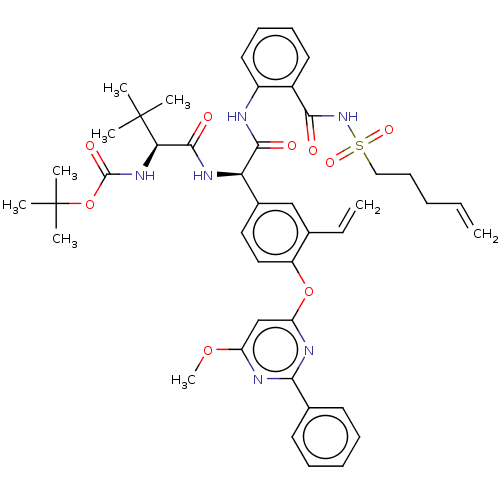 (CHEMBL3125047) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037503 (CHEMBL3356701) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037501 (CHEMBL1170408) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037451 (CHEMBL3356714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037451 (CHEMBL3356714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037491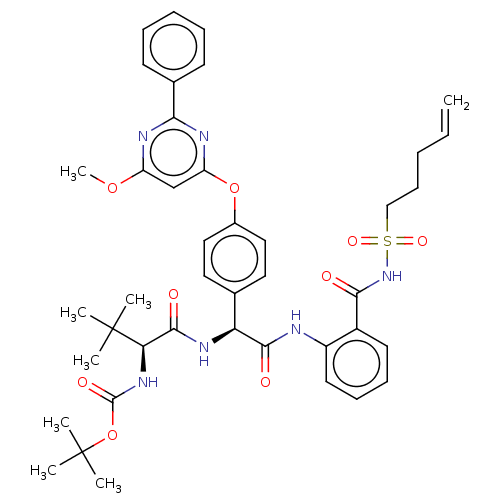 (CHEMBL3356708) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037494 (CHEMBL3356706) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037455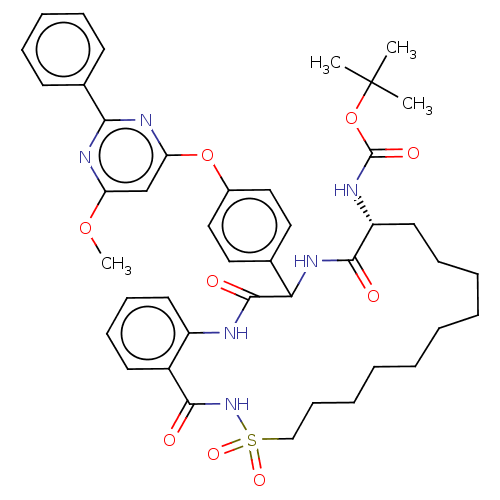 (CHEMBL3356716) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037445 (CHEMBL3356717) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037451 (CHEMBL3356714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV NS3/4A protease A156T mutant using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037495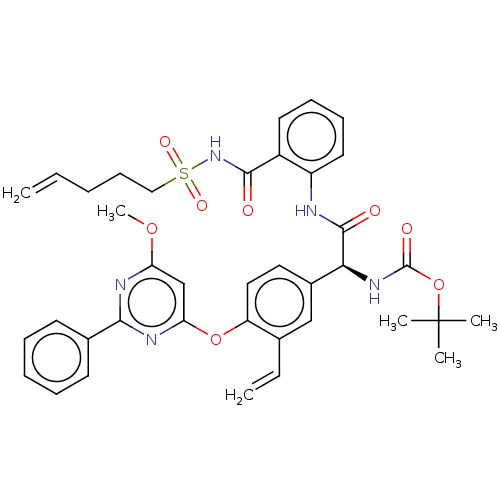 (CHEMBL3125171) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037456 (CHEMBL3356715) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037451 (CHEMBL3356714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV NS3/4A protease R155K mutant using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037498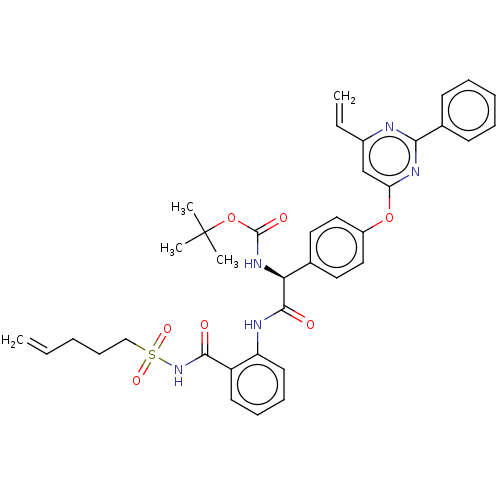 (CHEMBL3356703) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037493 (CHEMBL3356707) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037502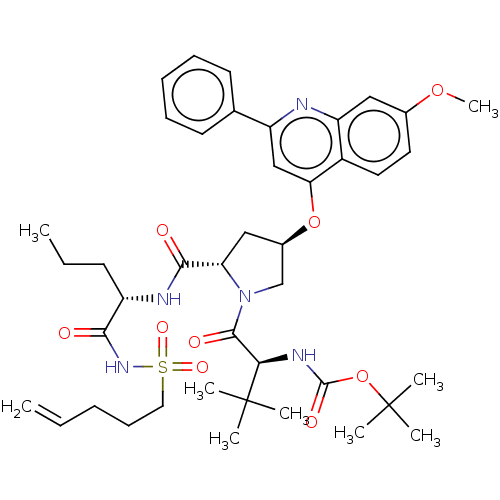 (CHEMBL1170421) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037499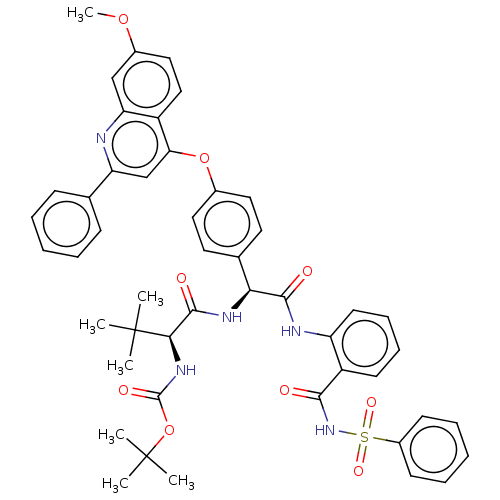 (CHEMBL3356702) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037496 (CHEMBL3356705) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037446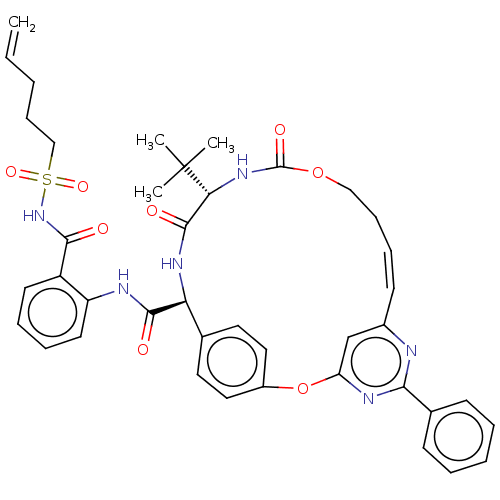 (CHEMBL3356718) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037466 (CHEMBL3356709) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037466 (CHEMBL3356709) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037451 (CHEMBL3356714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV NS3/4A protease D168V mutant using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037497 (CHEMBL3356704) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037458 (CHEMBL3356713) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037461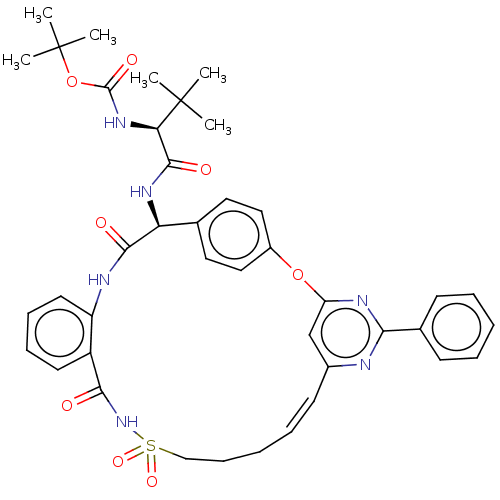 (CHEMBL3356711) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037500 (CHEMBL259478) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037460 (CHEMBL3356712) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037449 (CHEMBL3356719) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50037465 (CHEMBL3356710) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1a NS3/4A protease using Ac-DED(Edans)EEAbu-psi[COO]ASK(Dabcyl)-NH2 as substrate by FRET assay | Bioorg Med Chem 22: 6595-615 (2015) Article DOI: 10.1016/j.bmc.2014.10.010 BindingDB Entry DOI: 10.7270/Q2GX4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||