

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145681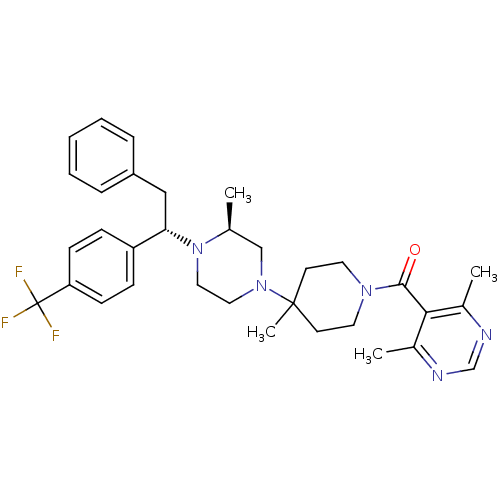 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685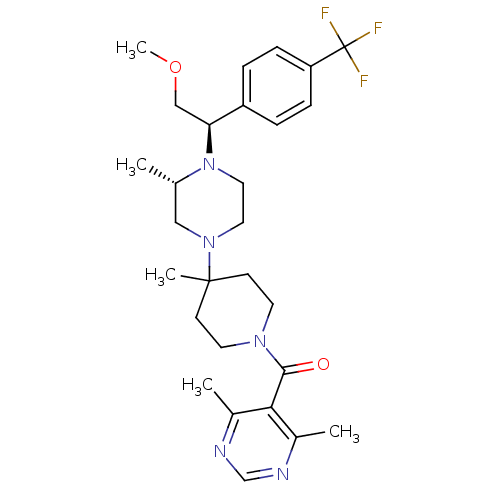 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145682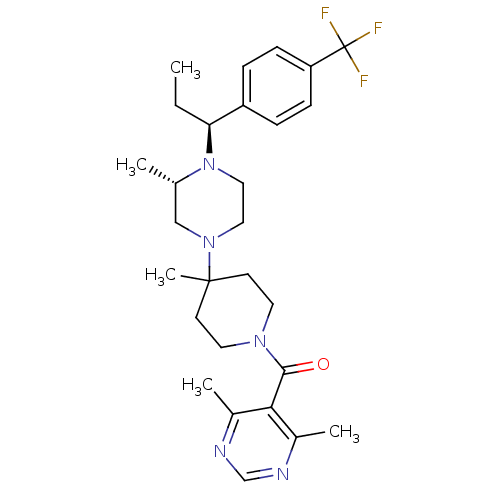 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 716 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145683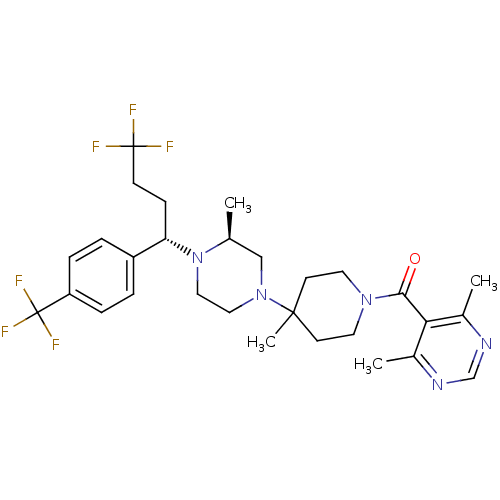 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145681 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145683 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50145682 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M3 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50145685 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic M1 receptor | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||