 Found 72 hits Enz. Inhib. hit(s) with all data for entry = 50045127
Found 72 hits Enz. Inhib. hit(s) with all data for entry = 50045127 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM21973
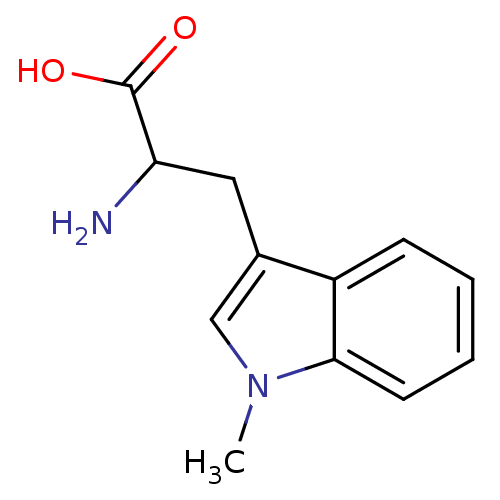
(1-Methyltryptophan, 1 | 2-amino-3-(1-methyl-1H-ind...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO using L-tryptophan as substrate |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046105
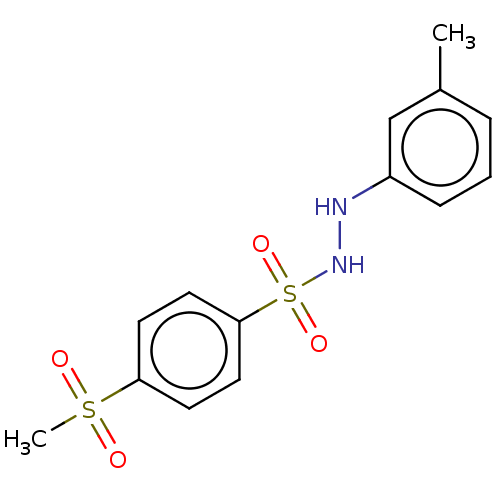
(CHEMBL3310843)Show InChI InChI=1S/C14H16N2O4S2/c1-11-4-3-5-12(10-11)15-16-22(19,20)14-8-6-13(7-9-14)21(2,17)18/h3-10,15-16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM80319

(MLS001171649 | N-[4-(m-toluidinosulfamoyl)phenyl]a...)Show InChI InChI=1S/C15H17N3O3S/c1-11-4-3-5-14(10-11)17-18-22(20,21)15-8-6-13(7-9-15)16-12(2)19/h3-10,17-18H,1-2H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046106
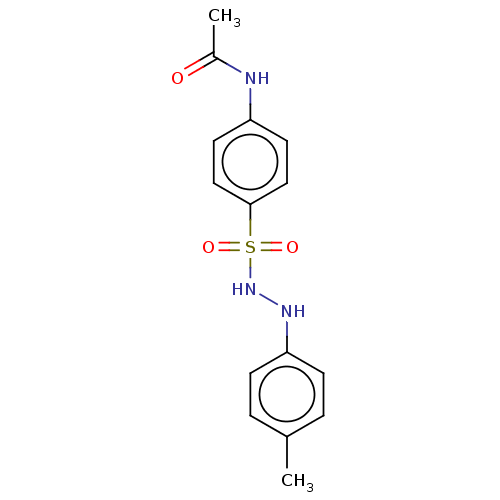
(CHEMBL3310847)Show InChI InChI=1S/C15H17N3O3S/c1-11-3-5-14(6-4-11)17-18-22(20,21)15-9-7-13(8-10-15)16-12(2)19/h3-10,17-18H,1-2H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046107
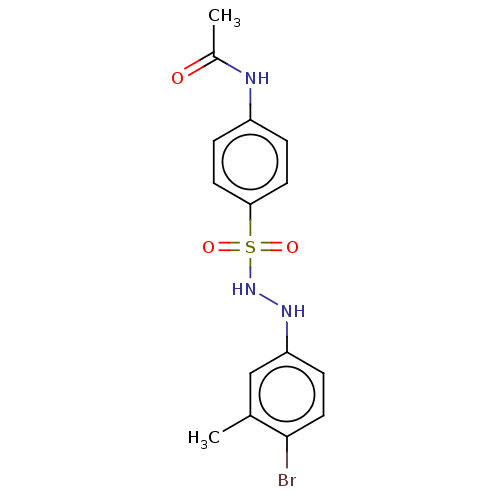
(CHEMBL3310860)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Br)c(C)c1 Show InChI InChI=1S/C15H16BrN3O3S/c1-10-9-13(5-8-15(10)16)18-19-23(21,22)14-6-3-12(4-7-14)17-11(2)20/h3-9,18-19H,1-2H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046108

(CHEMBL3310844)Show InChI InChI=1S/C14H13N3O2S/c1-11-3-2-4-13(9-11)16-17-20(18,19)14-7-5-12(10-15)6-8-14/h2-9,16-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046109
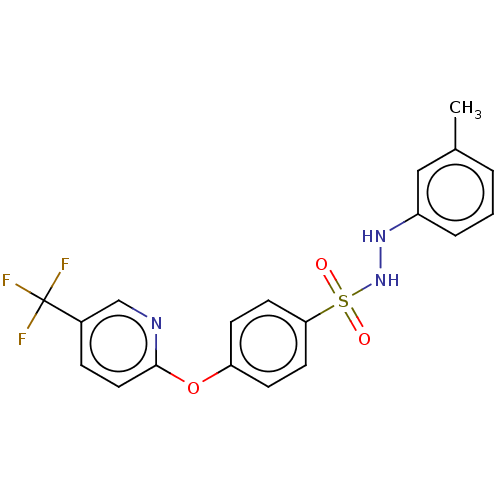
(CHEMBL3310845)Show SMILES Cc1cccc(NNS(=O)(=O)c2ccc(Oc3ccc(cn3)C(F)(F)F)cc2)c1 Show InChI InChI=1S/C19H16F3N3O3S/c1-13-3-2-4-15(11-13)24-25-29(26,27)17-8-6-16(7-9-17)28-18-10-5-14(12-23-18)19(20,21)22/h2-12,24-25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046110
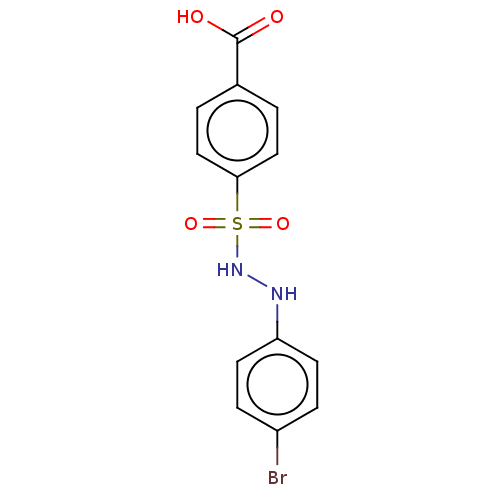
(CHEMBL3310981)Show InChI InChI=1S/C13H11BrN2O4S/c14-10-3-5-11(6-4-10)15-16-21(19,20)12-7-1-9(2-8-12)13(17)18/h1-8,15-16H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046125
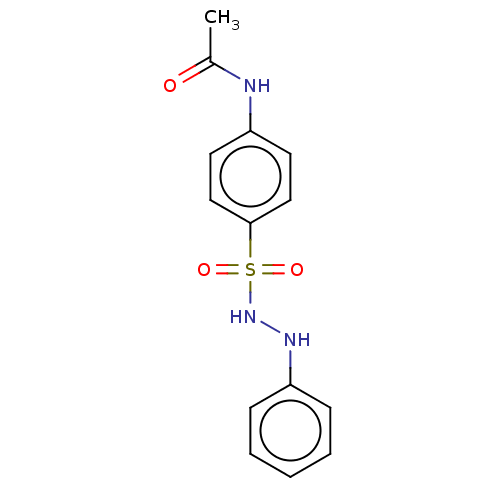
(CHEMBL3310846)Show InChI InChI=1S/C14H15N3O3S/c1-11(18)15-12-7-9-14(10-8-12)21(19,20)17-16-13-5-3-2-4-6-13/h2-10,16-17H,1H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046127
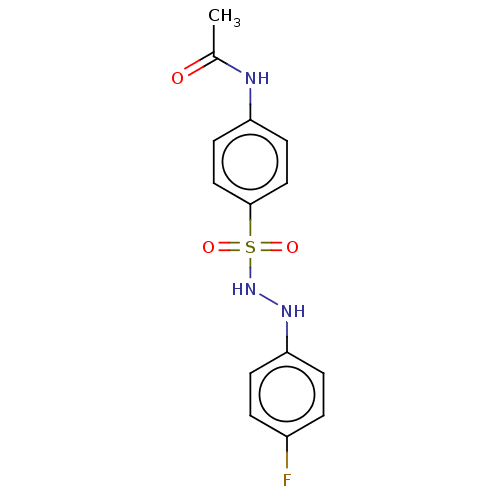
(CHEMBL3310852)Show InChI InChI=1S/C14H14FN3O3S/c1-10(19)16-12-6-8-14(9-7-12)22(20,21)18-17-13-4-2-11(15)3-5-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046136
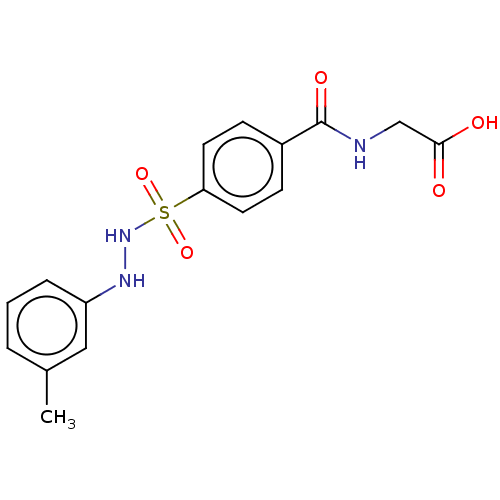
(CHEMBL3310298)Show SMILES Cc1cccc(NNS(=O)(=O)c2ccc(cc2)C(=O)NCC(O)=O)c1 Show InChI InChI=1S/C16H17N3O5S/c1-11-3-2-4-13(9-11)18-19-25(23,24)14-7-5-12(6-8-14)16(22)17-10-15(20)21/h2-9,18-19H,10H2,1H3,(H,17,22)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046140
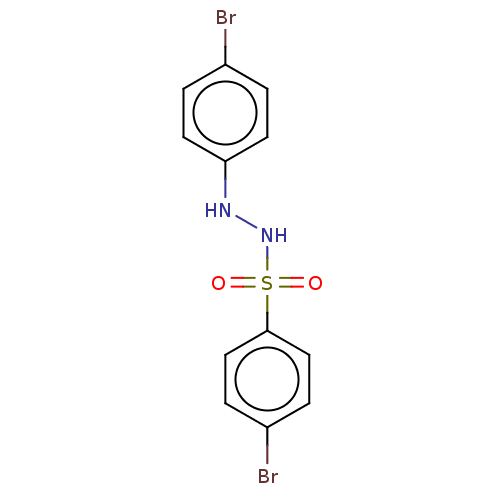
(CHEMBL3310974)Show InChI InChI=1S/C12H10Br2N2O2S/c13-9-1-5-11(6-2-9)15-16-19(17,18)12-7-3-10(14)4-8-12/h1-8,15-16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046142

(CHEMBL3310858)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(F)c(Cl)c1 Show InChI InChI=1S/C14H13ClFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-14(16)13(15)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046143
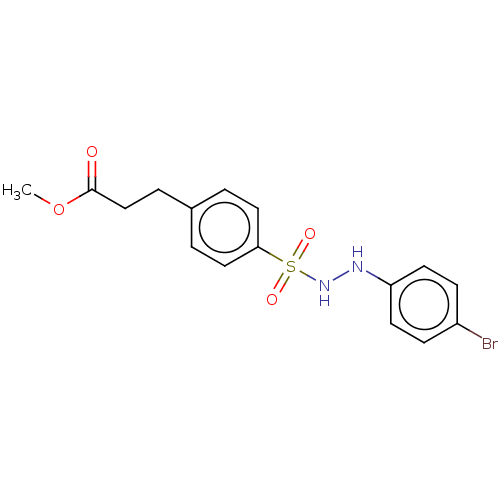
(CHEMBL3310977)Show InChI InChI=1S/C16H17BrN2O4S/c1-23-16(20)11-4-12-2-9-15(10-3-12)24(21,22)19-18-14-7-5-13(17)6-8-14/h2-3,5-10,18-19H,4,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046144
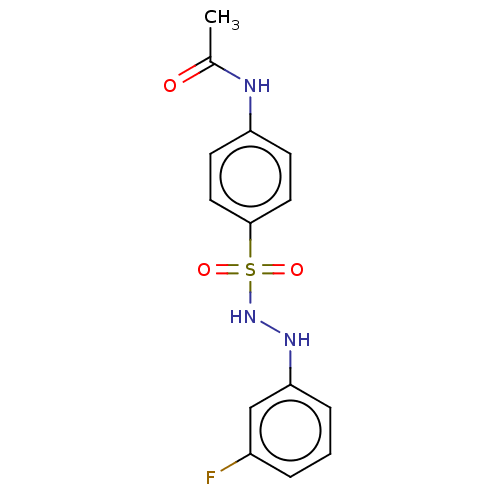
(CHEMBL3310855)Show InChI InChI=1S/C14H14FN3O3S/c1-10(19)16-12-5-7-14(8-6-12)22(20,21)18-17-13-4-2-3-11(15)9-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046146
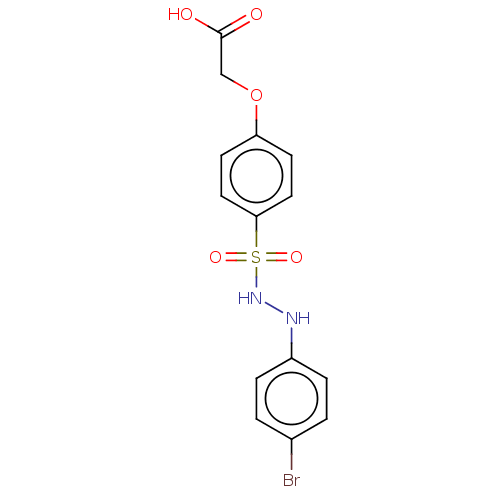
(CHEMBL3310980)Show InChI InChI=1S/C14H13BrN2O5S/c15-10-1-3-11(4-2-10)16-17-23(20,21)13-7-5-12(6-8-13)22-9-14(18)19/h1-8,16-17H,9H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046147
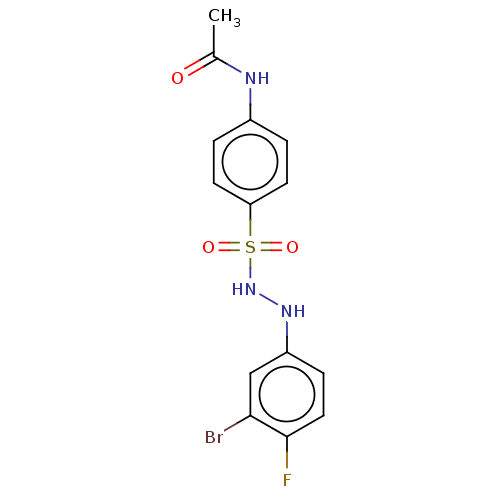
(CHEMBL3310857)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(F)c(Br)c1 Show InChI InChI=1S/C14H13BrFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-14(16)13(15)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046141

(CHEMBL3310854)Show InChI InChI=1S/C14H14BrN3O3S/c1-10(19)16-12-6-8-14(9-7-12)22(20,21)18-17-13-4-2-11(15)3-5-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046149

(CHEMBL3310978)Show InChI InChI=1S/C16H18BrN3O3S/c1-2-3-16(21)18-13-8-10-15(11-9-13)24(22,23)20-19-14-6-4-12(17)5-7-14/h4-11,19-20H,2-3H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046151
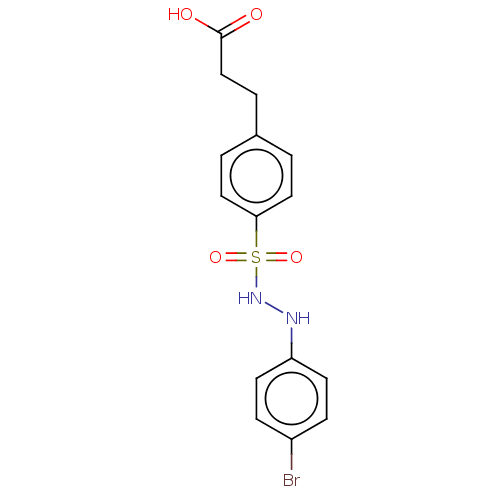
(CHEMBL3310979)Show InChI InChI=1S/C15H15BrN2O4S/c16-12-4-6-13(7-5-12)17-18-23(21,22)14-8-1-11(2-9-14)3-10-15(19)20/h1-2,4-9,17-18H,3,10H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046152
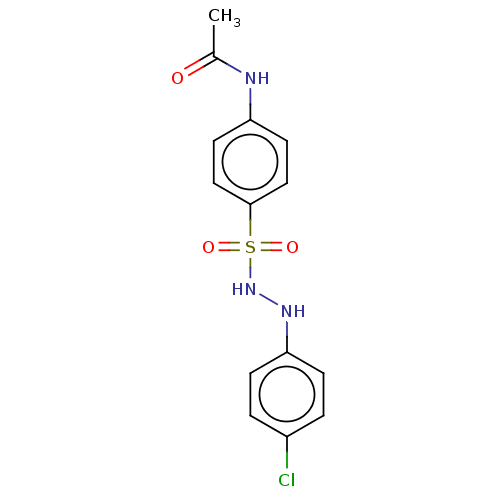
(CHEMBL3310853)Show InChI InChI=1S/C14H14ClN3O3S/c1-10(19)16-12-6-8-14(9-7-12)22(20,21)18-17-13-4-2-11(15)3-5-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046104
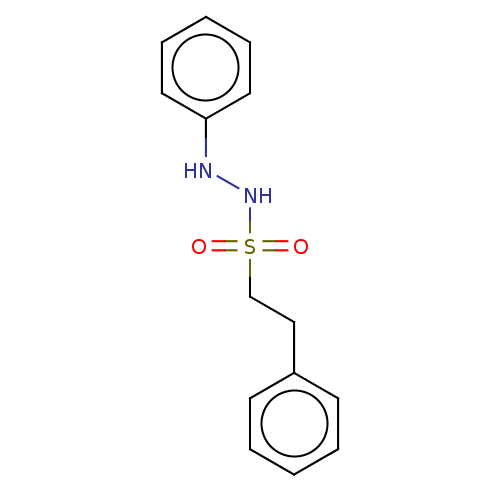
(CHEMBL3310297)Show InChI InChI=1S/C14H16N2O2S/c17-19(18,12-11-13-7-3-1-4-8-13)16-15-14-9-5-2-6-10-14/h1-10,15-16H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046148
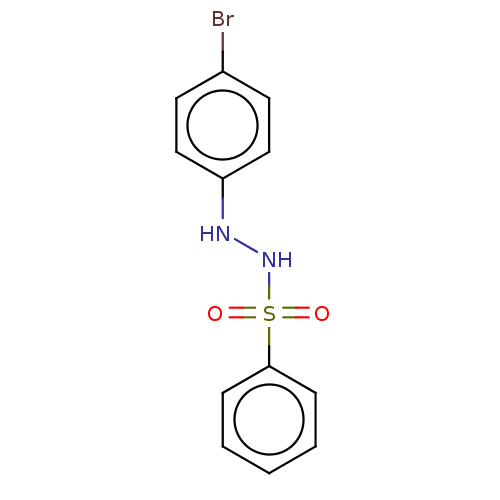
(CHEMBL3310973)Show InChI InChI=1S/C12H11BrN2O2S/c13-10-6-8-11(9-7-10)14-15-18(16,17)12-4-2-1-3-5-12/h1-9,14-15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046150

(CHEMBL3310976)Show InChI InChI=1S/C13H10BrN3O2S/c14-11-3-5-12(6-4-11)16-17-20(18,19)13-7-1-10(9-15)2-8-13/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046139
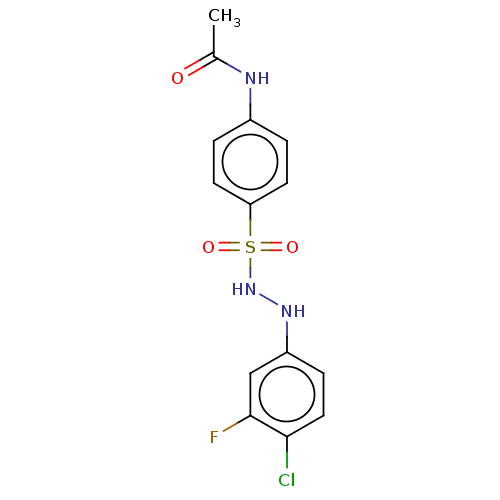
(CHEMBL3310861)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Cl)c(F)c1 Show InChI InChI=1S/C14H13ClFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-13(15)14(16)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046154

(CHEMBL3310859)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Br)c(F)c1 Show InChI InChI=1S/C14H13BrFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-13(15)14(16)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046155
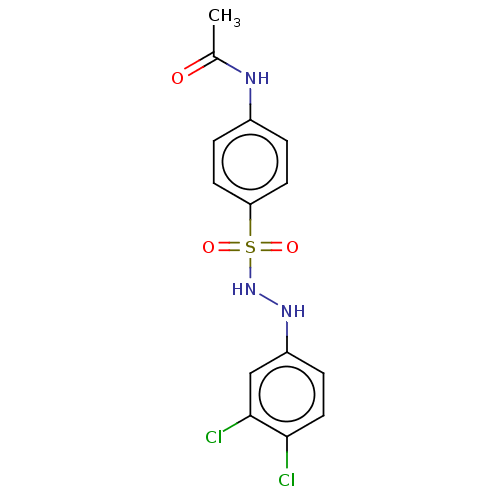
(CHEMBL3310864)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C14H13Cl2N3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-13(15)14(16)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046156
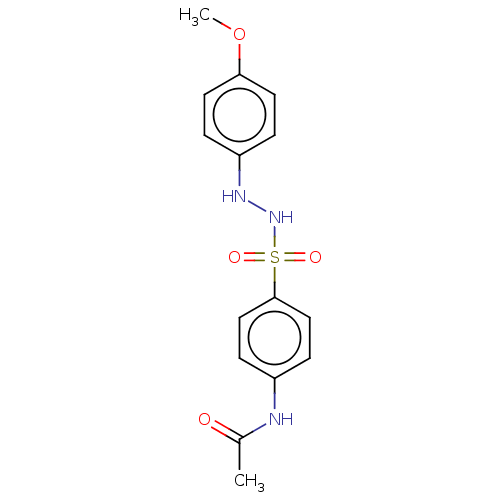
(CHEMBL3310848)Show InChI InChI=1S/C15H17N3O4S/c1-11(19)16-12-5-9-15(10-6-12)23(20,21)18-17-13-3-7-14(22-2)8-4-13/h3-10,17-18H,1-2H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046157
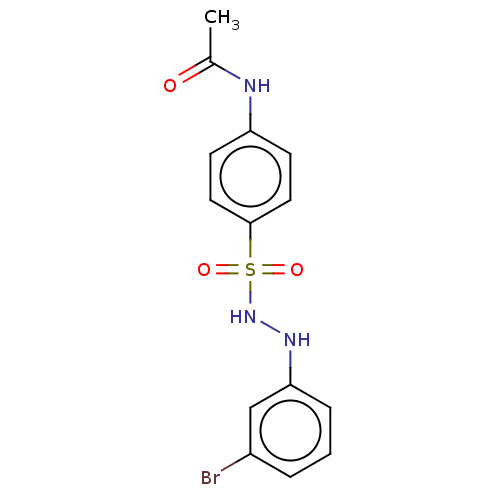
(CHEMBL3310856)Show InChI InChI=1S/C14H14BrN3O3S/c1-10(19)16-12-5-7-14(8-6-12)22(20,21)18-17-13-4-2-3-11(15)9-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046158
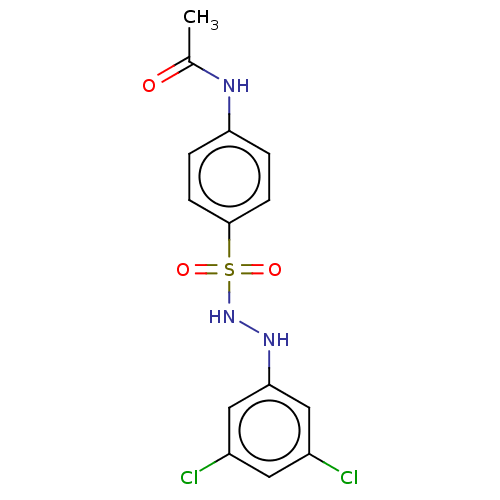
(CHEMBL3310972)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C14H13Cl2N3O3S/c1-9(20)17-12-2-4-14(5-3-12)23(21,22)19-18-13-7-10(15)6-11(16)8-13/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046159
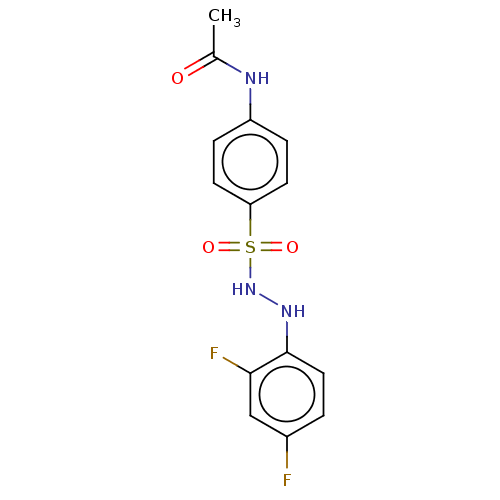
(CHEMBL3310863)Show InChI InChI=1S/C14H13F2N3O3S/c1-9(20)17-11-3-5-12(6-4-11)23(21,22)19-18-14-7-2-10(15)8-13(14)16/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046160

(CHEMBL3310975)Show InChI InChI=1S/C13H13BrN2O3S/c1-19-12-6-8-13(9-7-12)20(17,18)16-15-11-4-2-10(14)3-5-11/h2-9,15-16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046153
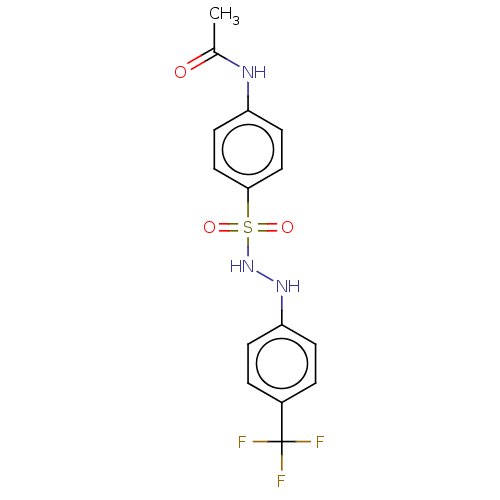
(CHEMBL3310851)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C15H14F3N3O3S/c1-10(22)19-12-6-8-14(9-7-12)25(23,24)21-20-13-4-2-11(3-5-13)15(16,17)18/h2-9,20-21H,1H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046162
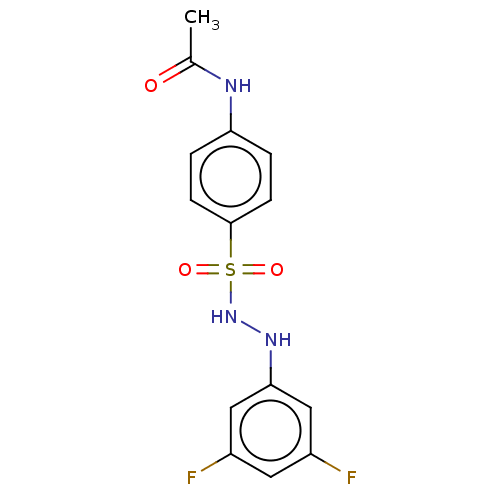
(CHEMBL3310862)Show InChI InChI=1S/C14H13F2N3O3S/c1-9(20)17-12-2-4-14(5-3-12)23(21,22)19-18-13-7-10(15)6-11(16)8-13/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046163
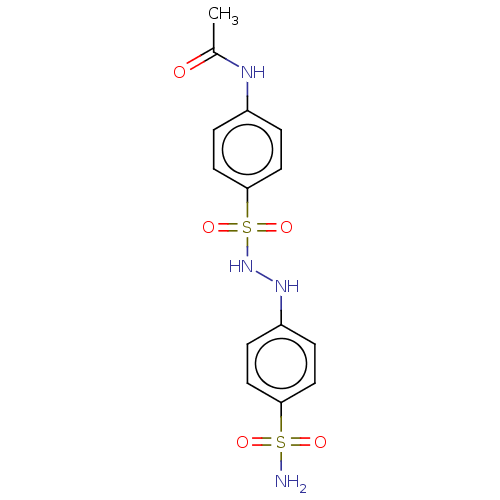
(CHEMBL3310850)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C14H16N4O5S2/c1-10(19)16-11-2-8-14(9-3-11)25(22,23)18-17-12-4-6-13(7-5-12)24(15,20)21/h2-9,17-18H,1H3,(H,16,19)(H2,15,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046164
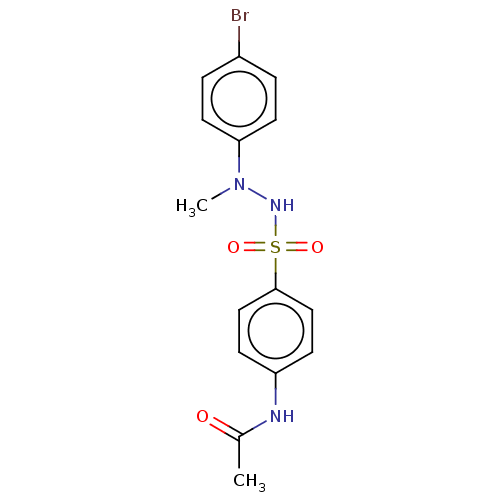
(CHEMBL3310982)Show SMILES CN(NS(=O)(=O)c1ccc(NC(C)=O)cc1)c1ccc(Br)cc1 Show InChI InChI=1S/C15H16BrN3O3S/c1-11(20)17-13-5-9-15(10-6-13)23(21,22)18-19(2)14-7-3-12(16)4-8-14/h3-10,18H,1-2H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046161
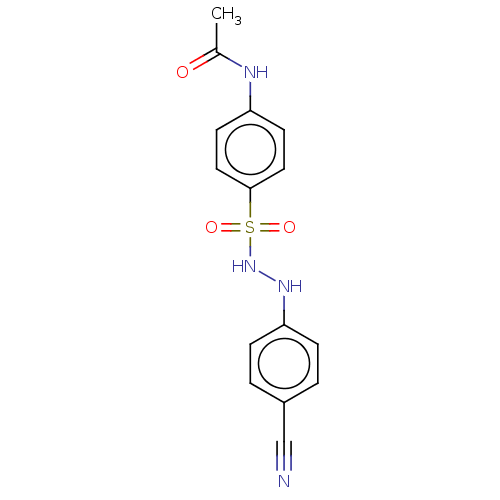
(CHEMBL3310849)Show InChI InChI=1S/C15H14N4O3S/c1-11(20)17-13-6-8-15(9-7-13)23(21,22)19-18-14-4-2-12(10-16)3-5-14/h2-9,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO expressed in Escherichia coli using N-formylkynurenine as substrate incubated for 1 hr prior to NaOH addition mea... |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046104

(CHEMBL3310297)Show InChI InChI=1S/C14H16N2O2S/c17-19(18,12-11-13-7-3-1-4-8-13)16-15-14-9-5-2-6-10-14/h1-10,15-16H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046139

(CHEMBL3310861)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Cl)c(F)c1 Show InChI InChI=1S/C14H13ClFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-13(15)14(16)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046141

(CHEMBL3310854)Show InChI InChI=1S/C14H14BrN3O3S/c1-10(19)16-12-6-8-14(9-7-12)22(20,21)18-17-13-4-2-11(15)3-5-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046148

(CHEMBL3310973)Show InChI InChI=1S/C12H11BrN2O2S/c13-10-6-8-11(9-7-10)14-15-18(16,17)12-4-2-1-3-5-12/h1-9,14-15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 128 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046107

(CHEMBL3310860)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Br)c(C)c1 Show InChI InChI=1S/C15H16BrN3O3S/c1-10-9-13(5-8-15(10)16)18-19-23(21,22)14-6-3-12(4-7-14)17-11(2)20/h3-9,18-19H,1-2H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046150

(CHEMBL3310976)Show InChI InChI=1S/C13H10BrN3O2S/c14-11-3-5-12(6-4-11)16-17-20(18,19)13-7-1-10(9-15)2-8-13/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 134 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046152

(CHEMBL3310853)Show InChI InChI=1S/C14H14ClN3O3S/c1-10(19)16-12-6-8-14(9-7-12)22(20,21)18-17-13-4-2-11(15)3-5-13/h2-9,17-18H,1H3,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046146

(CHEMBL3310980)Show InChI InChI=1S/C14H13BrN2O5S/c15-10-1-3-11(4-2-10)16-17-23(20,21)13-7-5-12(6-8-13)22-9-14(18)19/h1-8,16-17H,9H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 152 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046110

(CHEMBL3310981)Show InChI InChI=1S/C13H11BrN2O4S/c14-10-3-5-11(6-4-10)15-16-21(19,20)12-7-1-9(2-8-12)13(17)18/h1-8,15-16H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 172 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046153

(CHEMBL3310851)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C15H14F3N3O3S/c1-10(22)19-12-6-8-14(9-7-12)25(23,24)21-20-13-4-2-11(3-5-13)15(16,17)18/h2-9,20-21H,1H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 181 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046151

(CHEMBL3310979)Show InChI InChI=1S/C15H15BrN2O4S/c16-12-4-6-13(7-5-12)17-18-23(21,22)14-8-1-11(2-9-14)3-10-15(19)20/h1-2,4-9,17-18H,3,10H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 183 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046154

(CHEMBL3310859)Show SMILES CC(=O)Nc1ccc(cc1)S(=O)(=O)NNc1ccc(Br)c(F)c1 Show InChI InChI=1S/C14H13BrFN3O3S/c1-9(20)17-10-2-5-12(6-3-10)23(21,22)19-18-11-4-7-13(15)14(16)8-11/h2-8,18-19H,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 187 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50046149

(CHEMBL3310978)Show InChI InChI=1S/C16H18BrN3O3S/c1-2-3-16(21)18-13-8-10-15(11-9-13)24(22,23)20-19-14-6-4-12(17)5-7-14/h4-11,19-20H,2-3H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 187 | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of IDO in human HeLa cells after 24 hrs by spectrophotometry |
Bioorg Med Chem Lett 24: 3403-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.084
BindingDB Entry DOI: 10.7270/Q23R0VH6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data