 Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50045236
Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50045236 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50051818
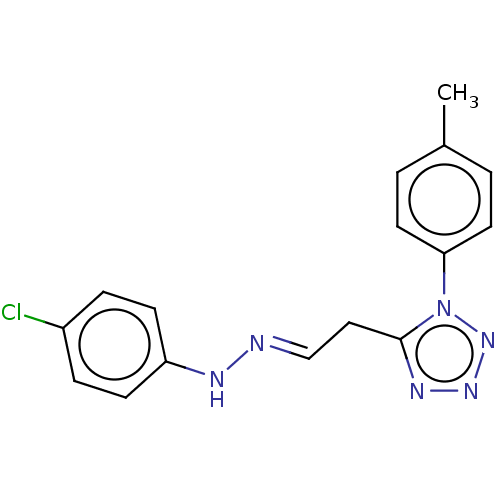
(CHEMBL3318333)Show InChI InChI=1S/C16H15ClN6/c1-12-2-8-15(9-3-12)23-16(20-21-22-23)10-11-18-19-14-6-4-13(17)5-7-14/h2-9,11,19H,10H2,1H3/b18-11+ | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051845
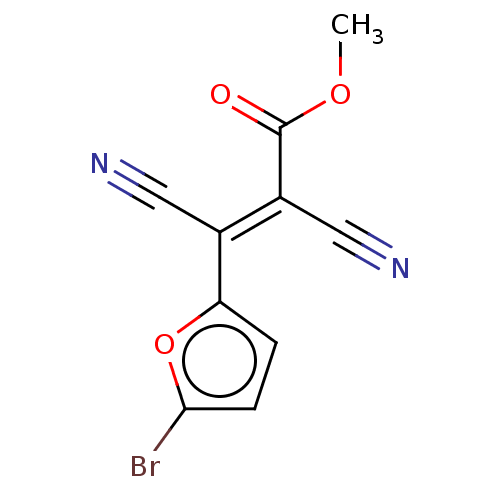
(CHEMBL3318327)Show InChI InChI=1S/C10H5BrN2O3/c1-15-10(14)7(5-13)6(4-12)8-2-3-9(11)16-8/h2-3H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051846
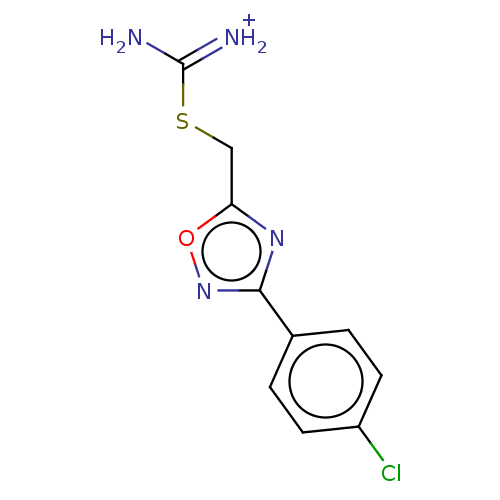
(CHEMBL3358454)Show InChI InChI=1S/C10H9ClN4OS/c11-7-3-1-6(2-4-7)9-14-8(16-15-9)5-17-10(12)13/h1-4H,5H2,(H3,12,13)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051847
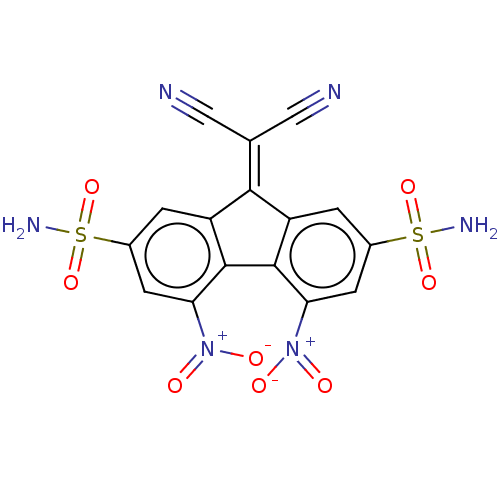
(CHEMBL3318328)Show SMILES [#7]S(=O)(=O)c1cc2\[#6](=[#6](\C#N)C#N)-c3cc(cc(c3-c2c(c1)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O)S([#7])(=O)=O Show InChI InChI=1S/C16H8N6O8S2/c17-5-7(6-18)14-10-1-8(31(19,27)28)3-12(21(23)24)15(10)16-11(14)2-9(32(20,29)30)4-13(16)22(25)26/h1-4H,(H2,19,27,28)(H2,20,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051847

(CHEMBL3318328)Show SMILES [#7]S(=O)(=O)c1cc2\[#6](=[#6](\C#N)C#N)-c3cc(cc(c3-c2c(c1)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O)S([#7])(=O)=O Show InChI InChI=1S/C16H8N6O8S2/c17-5-7(6-18)14-10-1-8(31(19,27)28)3-12(21(23)24)15(10)16-11(14)2-9(32(20,29)30)4-13(16)22(25)26/h1-4H,(H2,19,27,28)(H2,20,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051846

(CHEMBL3358454)Show InChI InChI=1S/C10H9ClN4OS/c11-7-3-1-6(2-4-7)9-14-8(16-15-9)5-17-10(12)13/h1-4H,5H2,(H3,12,13)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051847

(CHEMBL3318328)Show SMILES [#7]S(=O)(=O)c1cc2\[#6](=[#6](\C#N)C#N)-c3cc(cc(c3-c2c(c1)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O)S([#7])(=O)=O Show InChI InChI=1S/C16H8N6O8S2/c17-5-7(6-18)14-10-1-8(31(19,27)28)3-12(21(23)24)15(10)16-11(14)2-9(32(20,29)30)4-13(16)22(25)26/h1-4H,(H2,19,27,28)(H2,20,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051848
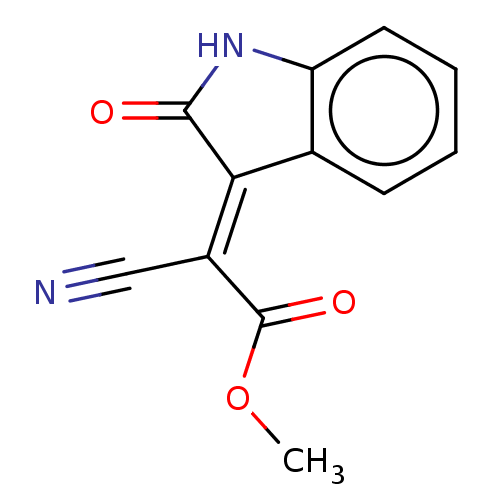
(CHEMBL3318329)Show InChI InChI=1S/C12H8N2O3/c1-17-12(16)8(6-13)10-7-4-2-3-5-9(7)14-11(10)15/h2-5H,1H3,(H,14,15)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50051846

(CHEMBL3358454)Show InChI InChI=1S/C10H9ClN4OS/c11-7-3-1-6(2-4-7)9-14-8(16-15-9)5-17-10(12)13/h1-4H,5H2,(H3,12,13)/p+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31772
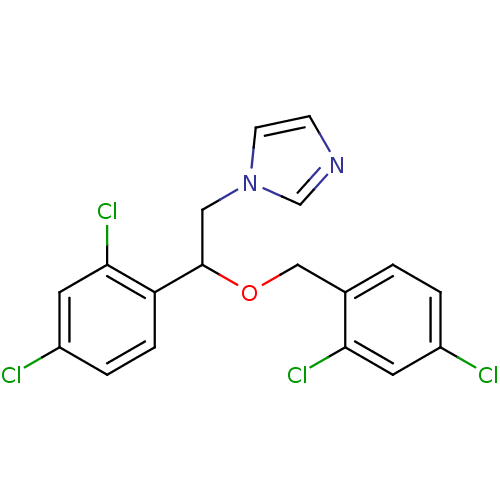
(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Mus musculus) | BDBM50051818

(CHEMBL3318333)Show InChI InChI=1S/C16H15ClN6/c1-12-2-8-15(9-3-12)23-16(20-21-22-23)10-11-18-19-14-6-4-13(17)5-7-14/h2-9,11,19H,10H2,1H3/b18-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of mouse TDO expressed in mouse P815B cells using L-tryptophan substrate incubated for 24 hrs by HPLC based cellular assay |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50247616

(2-(3-chloro-2-fluorophenyl)isothiazol-3(2H)-one | ...)Show InChI InChI=1S/C9H5ClFNOS/c10-6-2-1-3-7(9(6)11)12-8(13)4-5-14-12/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM92508

(DP 00477, 3)Show SMILES FC(F)(F)c1cccc(NC(=O)C(C#N)C(=S)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C17H11ClF3N3OS/c18-13-6-1-2-7-14(13)24-16(26)12(9-22)15(25)23-11-5-3-4-10(8-11)17(19,20)21/h1-8,12H,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31770
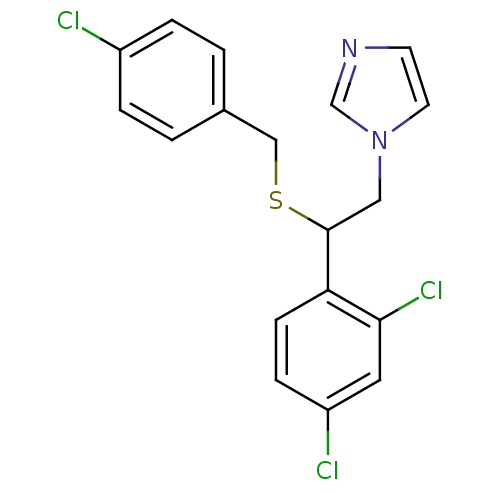
(Exelderm | Sulconazole Nitrate | cid_65495 | sulco...)Show InChI InChI=1S/C18H15Cl3N2S/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31772

(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM92508

(DP 00477, 3)Show SMILES FC(F)(F)c1cccc(NC(=O)C(C#N)C(=S)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C17H11ClF3N3OS/c18-13-6-1-2-7-14(13)24-16(26)12(9-22)15(25)23-11-5-3-4-10(8-11)17(19,20)21/h1-8,12H,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051845

(CHEMBL3318327)Show InChI InChI=1S/C10H5BrN2O3/c1-15-10(14)7(5-13)6(4-12)8-2-3-9(11)16-8/h2-3H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31773
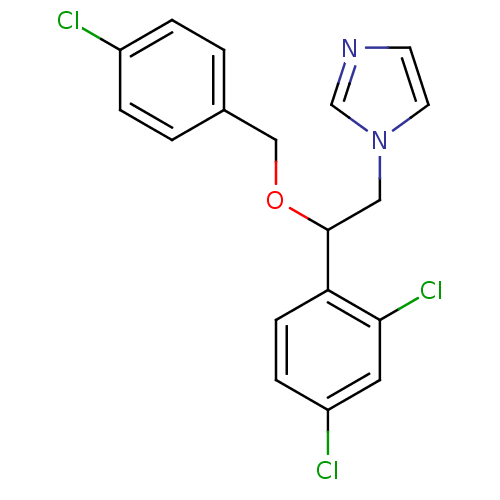
(ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...)Show InChI InChI=1S/C18H15Cl3N2O/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051842
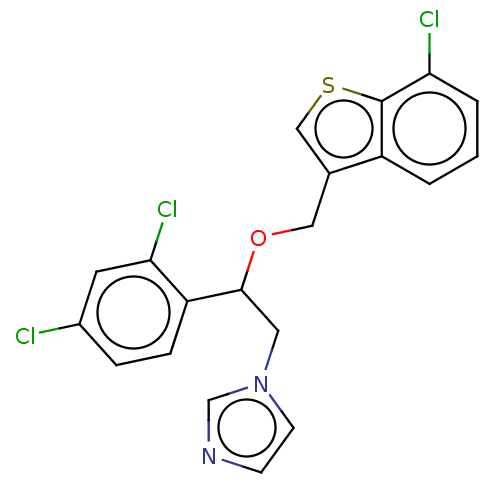
(CHEBI:83682 | Demofix | Ertaczo | Sertaconazole)Show SMILES Clc1ccc(C(Cn2ccnc2)OCc2csc3c(Cl)cccc23)c(Cl)c1 Show InChI InChI=1S/C20H15Cl3N2OS/c21-14-4-5-16(18(23)8-14)19(9-25-7-6-24-12-25)26-10-13-11-27-20-15(13)2-1-3-17(20)22/h1-8,11-12,19H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50247616

(2-(3-chloro-2-fluorophenyl)isothiazol-3(2H)-one | ...)Show InChI InChI=1S/C9H5ClFNOS/c10-6-2-1-3-7(9(6)11)12-8(13)4-5-14-12/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051817

(CHEMBL3318332)Show InChI InChI=1S/C12H11NO3S/c1-2-16-12(15)9-3-5-10(6-4-9)13-11(14)7-8-17-13/h3-8H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051849

(CHEMBL3318331)Show InChI InChI=1S/C13H11N5O2S/c14-11-9-8(6-21-11)10(12(19)16-15)17-18(13(9)20)7-4-2-1-3-5-7/h1-6H,14-15H2,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50105417

(CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...)Show InChI InChI=1S/C8H12N2/c9-10-7-6-8-4-2-1-3-5-8/h1-5,10H,6-7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051849

(CHEMBL3318331)Show InChI InChI=1S/C13H11N5O2S/c14-11-9-8(6-21-11)10(12(19)16-15)17-18(13(9)20)7-4-2-1-3-5-7/h1-6H,14-15H2,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051817

(CHEMBL3318332)Show InChI InChI=1S/C12H11NO3S/c1-2-16-12(15)9-3-5-10(6-4-9)13-11(14)7-8-17-13/h3-8H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051843

(CHEBI:83829 | Enilconazole | R-23979)Show InChI InChI=1S/C14H14Cl2N2O/c1-2-7-19-14(9-18-6-5-17-10-18)12-4-3-11(15)8-13(12)16/h2-6,8,10,14H,1,7,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50128548
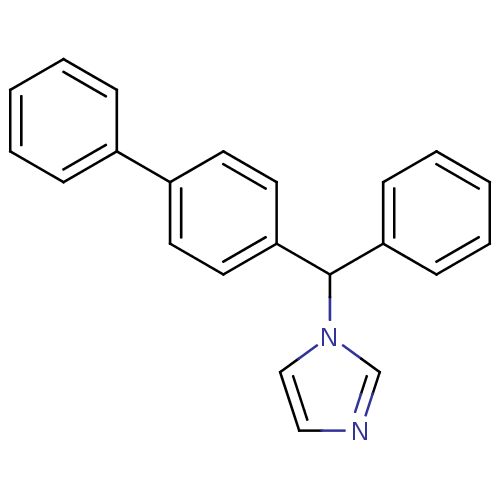
(1-(1-Biphenyl-4-yl-2-phenyl-methyl)-1H-imidazole |...)Show InChI InChI=1S/C22H18N2/c1-3-7-18(8-4-1)19-11-13-21(14-12-19)22(24-16-15-23-17-24)20-9-5-2-6-10-20/h1-17,22H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31771
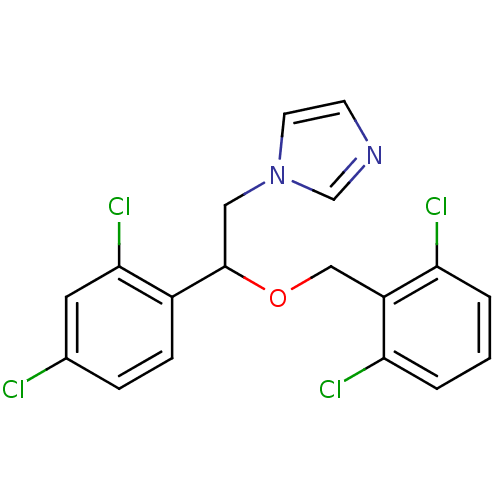
(Isoconazole | cid_3760)Show SMILES Clc1ccc(C(Cn2ccnc2)OCc2c(Cl)cccc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-12-4-5-13(17(22)8-12)18(9-24-7-6-23-11-24)25-10-14-15(20)2-1-3-16(14)21/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051844
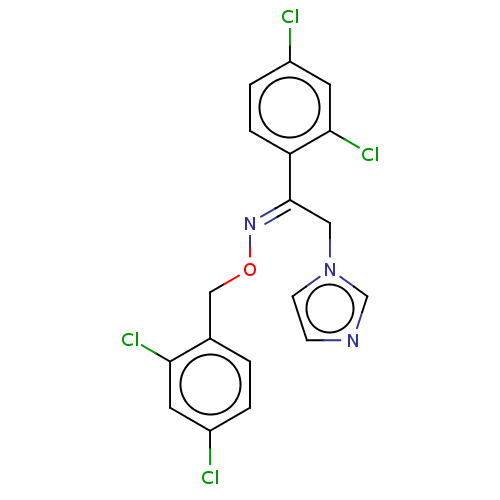
(CHEBI:7825 | Oxiconazole | Oxistat | Oxizole)Show SMILES Clc1ccc(CO\N=C(/Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H13Cl4N3O/c19-13-2-1-12(16(21)7-13)10-26-24-18(9-25-6-5-23-11-25)15-4-3-14(20)8-17(15)22/h1-8,11H,9-10H2/b24-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051818

(CHEMBL3318333)Show InChI InChI=1S/C16H15ClN6/c1-12-2-8-15(9-3-12)23-16(20-21-22-23)10-11-18-19-14-6-4-13(17)5-7-14/h2-9,11,19H,10H2,1H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051818

(CHEMBL3318333)Show InChI InChI=1S/C16H15ClN6/c1-12-2-8-15(9-3-12)23-16(20-21-22-23)10-11-18-19-14-6-4-13(17)5-7-14/h2-9,11,19H,10H2,1H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051818

(CHEMBL3318333)Show InChI InChI=1S/C16H15ClN6/c1-12-2-8-15(9-3-12)23-16(20-21-22-23)10-11-18-19-14-6-4-13(17)5-7-14/h2-9,11,19H,10H2,1H3/b18-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM92508

(DP 00477, 3)Show SMILES FC(F)(F)c1cccc(NC(=O)C(C#N)C(=S)Nc2ccccc2Cl)c1 Show InChI InChI=1S/C17H11ClF3N3OS/c18-13-6-1-2-7-14(13)24-16(26)12(9-22)15(25)23-11-5-3-4-10(8-11)17(19,20)21/h1-8,12H,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051837
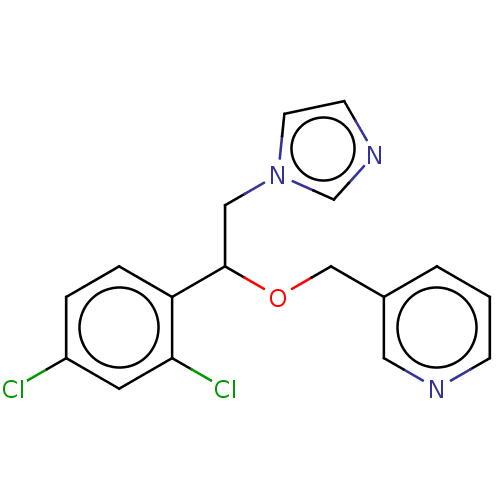
(CHEMBL3318359)Show InChI InChI=1S/C17H15Cl2N3O/c18-14-3-4-15(16(19)8-14)17(10-22-7-6-21-12-22)23-11-13-2-1-5-20-9-13/h1-9,12,17H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051817

(CHEMBL3318332)Show InChI InChI=1S/C12H11NO3S/c1-2-16-12(15)9-3-5-10(6-4-9)13-11(14)7-8-17-13/h3-8H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051838
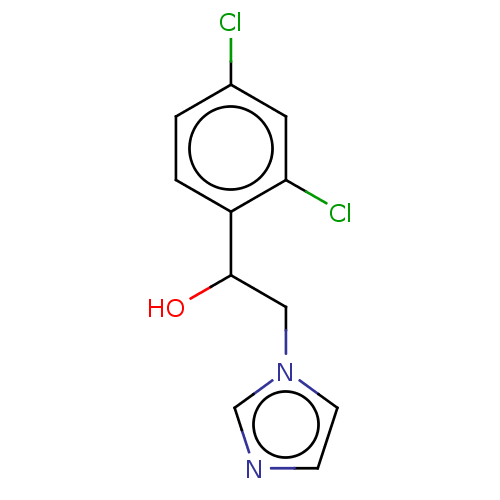
(CHEMBL1327)Show InChI InChI=1S/C11H10Cl2N2O/c12-8-1-2-9(10(13)5-8)11(16)6-15-4-3-14-7-15/h1-5,7,11,16H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051848

(CHEMBL3318329)Show InChI InChI=1S/C12H8N2O3/c1-17-12(16)8(6-13)10-7-4-2-3-5-9(7)14-11(10)15/h2-5H,1H3,(H,14,15)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051820

(CHEMBL3318358)Show InChI InChI=1S/C12H12Cl2N2O/c1-17-12(7-16-5-4-15-8-16)10-3-2-9(13)6-11(10)14/h2-6,8,12H,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051839
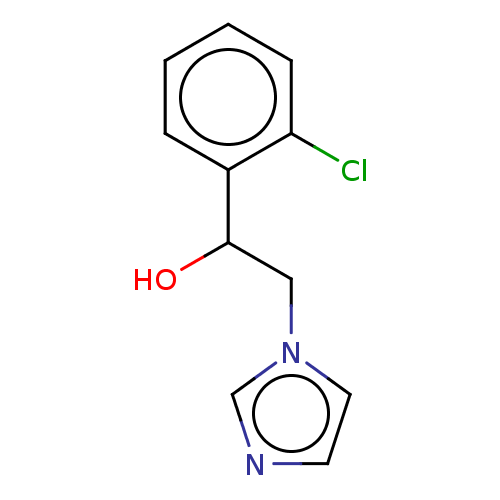
(CHEMBL3318360)Show InChI InChI=1S/C11H11ClN2O/c12-10-4-2-1-3-9(10)11(15)7-14-6-5-13-8-14/h1-6,8,11,15H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50247616

(2-(3-chloro-2-fluorophenyl)isothiazol-3(2H)-one | ...)Show InChI InChI=1S/C9H5ClFNOS/c10-6-2-1-3-7(9(6)11)12-8(13)4-5-14-12/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051841

(CHEMBL3318366)Show InChI InChI=1S/C10H8Cl2N2/c11-9-2-1-8(10(12)5-9)6-14-4-3-13-7-14/h1-5,7H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051819
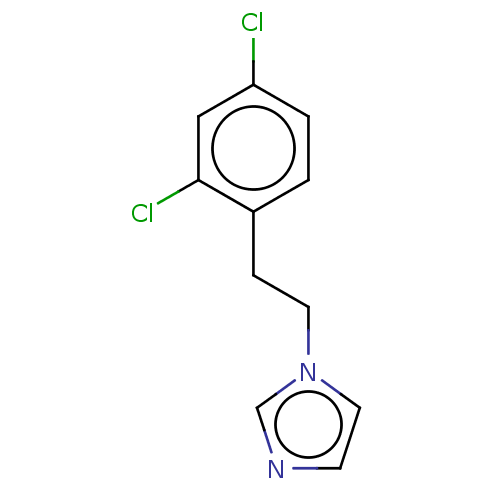
(CHEMBL3318357)Show InChI InChI=1S/C11H10Cl2N2/c12-10-2-1-9(11(13)7-10)3-5-15-6-4-14-8-15/h1-2,4,6-8H,3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50051840
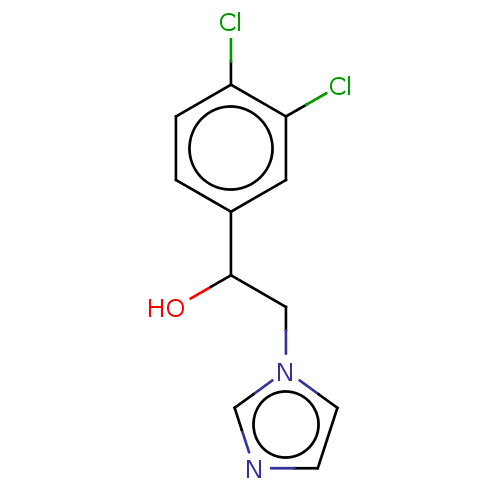
(CHEMBL3318361)Show InChI InChI=1S/C11H10Cl2N2O/c12-9-2-1-8(5-10(9)13)11(16)6-15-4-3-14-7-15/h1-5,7,11,16H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC |
Eur J Med Chem 84: 284-301 (2014)
Article DOI: 10.1016/j.ejmech.2014.06.078
BindingDB Entry DOI: 10.7270/Q21C1ZJV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data