 Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50015565
Found 25 hits Enz. Inhib. hit(s) with all data for entry = 50015565 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-lactamase
(Enterobacter cloacae) | BDBM50157689
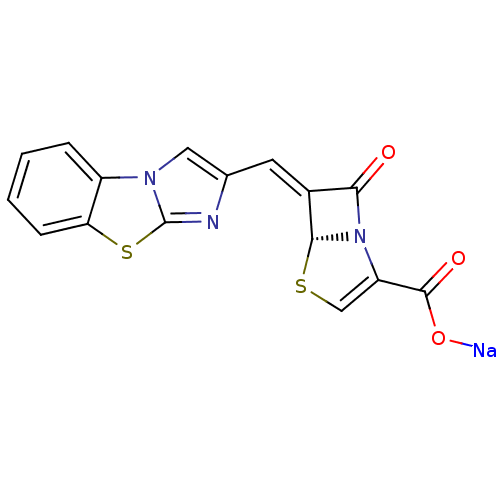
((5R,6Z)-6-(imidazo[2,1-b][1,3]benzothiazol-2-ylmet...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1ccccc21 |r,t:4| Show InChI InChI=1S/C16H9N3O3S2.Na/c20-13-9(14-19(13)11(7-23-14)15(21)22)5-8-6-18-10-3-1-2-4-12(10)24-16(18)17-8;/h1-7,14H,(H,21,22);/q;+1/p-1/b9-5-;/t14-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157688
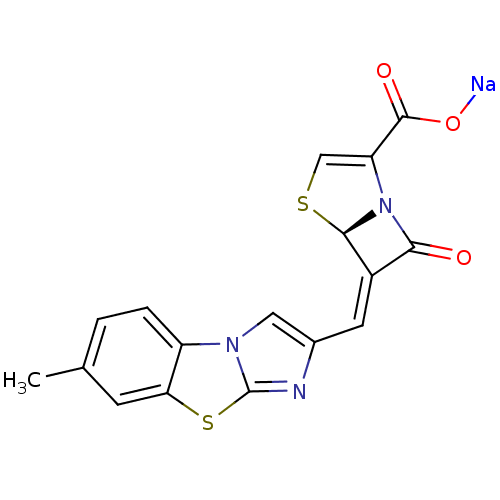
((5R,6Z)-6-[(7-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-2-3-11-13(4-8)25-17-18-9(6-19(11)17)5-10-14(21)20-12(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50149467
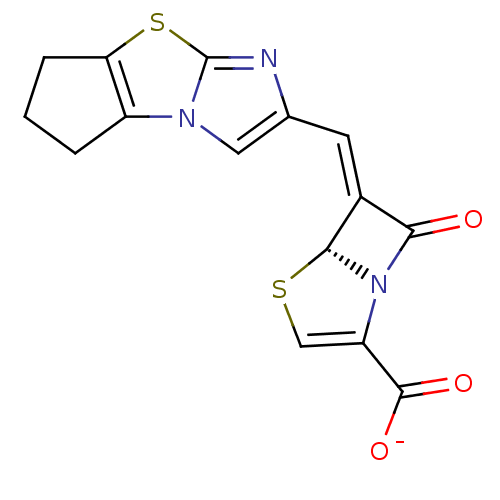
((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157694

((5R,6Z)-6-[(7-fluoroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Fc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C16H8FN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157687
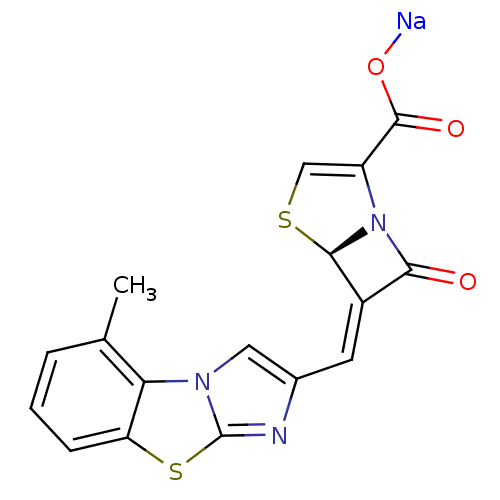
((5R,6Z)-6-[(5-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1cccc2sc3nc(\C=C4/[C@H]5SC=C(N5C4=O)C(=O)O[Na])cn3c12 |r,c:14| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-3-2-4-12-13(8)19-6-9(18-17(19)25-12)5-10-14(21)20-11(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157691
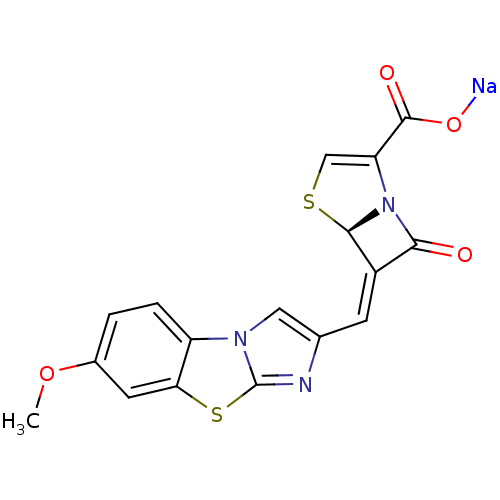
((5R,6Z)-6-[(7-methoxyimidazo[2,1-b][1,3]-benzothia...)Show SMILES COc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:17| Show InChI InChI=1S/C17H11N3O4S2.Na/c1-24-9-2-3-11-13(5-9)26-17-18-8(6-19(11)17)4-10-14(21)20-12(16(22)23)7-25-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-4-;/t15-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157688

((5R,6Z)-6-[(7-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-2-3-11-13(4-8)25-17-18-9(6-19(11)17)5-10-14(21)20-12(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157693
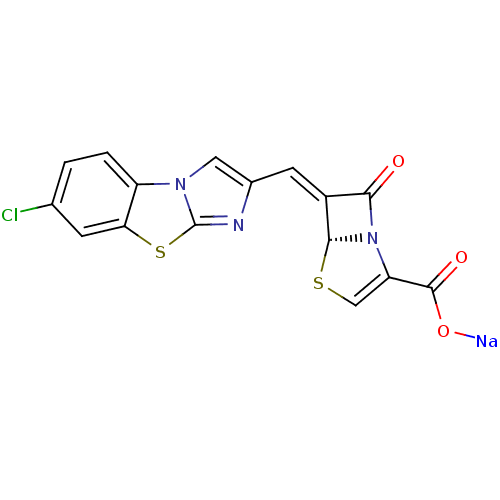
((5R,6Z)-6-[(7-chloroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1cc(Cl)ccc21 |r,t:4| Show InChI InChI=1S/C16H8ClN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157693

((5R,6Z)-6-[(7-chloroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1cc(Cl)ccc21 |r,t:4| Show InChI InChI=1S/C16H8ClN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157694

((5R,6Z)-6-[(7-fluoroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Fc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C16H8FN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157687

((5R,6Z)-6-[(5-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1cccc2sc3nc(\C=C4/[C@H]5SC=C(N5C4=O)C(=O)O[Na])cn3c12 |r,c:14| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-3-2-4-12-13(8)19-6-9(18-17(19)25-12)5-10-14(21)20-11(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157689

((5R,6Z)-6-(imidazo[2,1-b][1,3]benzothiazol-2-ylmet...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1ccccc21 |r,t:4| Show InChI InChI=1S/C16H9N3O3S2.Na/c20-13-9(14-19(13)11(7-23-14)15(21)22)5-8-6-18-10-3-1-2-4-12(10)24-16(18)17-8;/h1-7,14H,(H,21,22);/q;+1/p-1/b9-5-;/t14-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase SHV-1
(Klebsiella pneumoniae (Enterobacteria)) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli SHV1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157691

((5R,6Z)-6-[(7-methoxyimidazo[2,1-b][1,3]-benzothia...)Show SMILES COc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:17| Show InChI InChI=1S/C17H11N3O4S2.Na/c1-24-9-2-3-11-13(5-9)26-17-18-8(6-19(11)17)4-10-14(21)20-12(16(22)23)7-25-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-4-;/t15-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157693

((5R,6Z)-6-[(7-chloroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1cc(Cl)ccc21 |r,t:4| Show InChI InChI=1S/C16H8ClN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase TEM
(Escherichia coli) | BDBM50157692
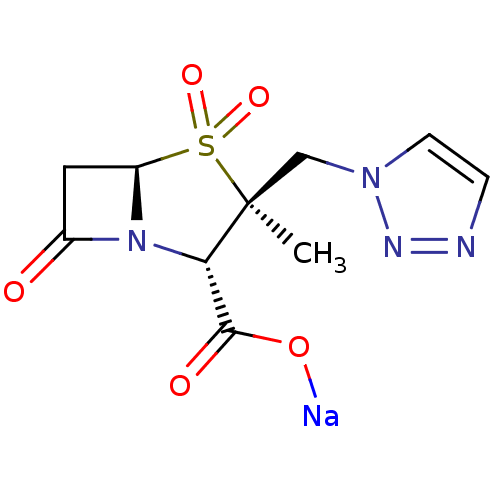
(CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(=O)O[Na] |r| Show InChI InChI=1S/C10H12N4O5S.Na/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19;/h2-3,7-8H,4-5H2,1H3,(H,16,17);/q;+1/p-1/t7-,8+,10+;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli TEM1 |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157688

((5R,6Z)-6-[(7-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-2-3-11-13(4-8)25-17-18-9(6-19(11)17)5-10-14(21)20-12(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50149467

((5R,6Z)-6-(6,7-dihydro-5H-cyclopenta-[d]imidazo[2,...)Show SMILES [O-]C(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c3CCCc3sc2n1 |t:3| Show InChI InChI=1S/C15H11N3O3S2/c19-12-8(13-18(12)10(6-22-13)14(20)21)4-7-5-17-9-2-1-3-11(9)23-15(17)16-7/h4-6,13H,1-3H2,(H,20,21)/p-1/b8-4-/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157691

((5R,6Z)-6-[(7-methoxyimidazo[2,1-b][1,3]-benzothia...)Show SMILES COc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:17| Show InChI InChI=1S/C17H11N3O4S2.Na/c1-24-9-2-3-11-13(5-9)26-17-18-8(6-19(11)17)4-10-14(21)20-12(16(22)23)7-25-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-4-;/t15-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157689

((5R,6Z)-6-(imidazo[2,1-b][1,3]benzothiazol-2-ylmet...)Show SMILES [Na]OC(=O)C1=CS[C@H]2N1C(=O)\C2=C\c1cn2c(n1)sc1ccccc21 |r,t:4| Show InChI InChI=1S/C16H9N3O3S2.Na/c20-13-9(14-19(13)11(7-23-14)15(21)22)5-8-6-18-10-3-1-2-4-12(10)24-16(18)17-8;/h1-7,14H,(H,21,22);/q;+1/p-1/b9-5-;/t14-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157687

((5R,6Z)-6-[(5-methylimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Cc1cccc2sc3nc(\C=C4/[C@H]5SC=C(N5C4=O)C(=O)O[Na])cn3c12 |r,c:14| Show InChI InChI=1S/C17H11N3O3S2.Na/c1-8-3-2-4-12-13(8)19-6-9(18-17(19)25-12)5-10-14(21)20-11(16(22)23)7-24-15(10)20;/h2-7,15H,1H3,(H,22,23);/q;+1/p-1/b10-5-;/t15-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157694

((5R,6Z)-6-[(7-fluoroimidazo[2,1-b][1,3]-benzothiaz...)Show SMILES Fc1ccc2c(c1)sc1nc(\C=C3/[C@H]4SC=C(N4C3=O)C(=O)O[Na])cn21 |r,c:16| Show InChI InChI=1S/C16H8FN3O3S2.Na/c17-7-1-2-10-12(3-7)25-16-18-8(5-19(10)16)4-9-13(21)20-11(15(22)23)6-24-14(9)20;/h1-6,14H,(H,22,23);/q;+1/p-1/b9-4-;/t14-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Enterobacter cloacae) | BDBM50157692

(CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(=O)O[Na] |r| Show InChI InChI=1S/C10H12N4O5S.Na/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19;/h2-3,7-8H,4-5H2,1H3,(H,16,17);/q;+1/p-1/t7-,8+,10+;/m1./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Enterobacter cloacae AmpC |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |
Metallo-beta-lactamase type 2
(Bacteroides fragilis) | BDBM50157692

(CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...)Show SMILES C[C@]1(Cn2ccnn2)[C@@H](N2[C@@H](CC2=O)S1(=O)=O)C(=O)O[Na] |r| Show InChI InChI=1S/C10H12N4O5S.Na/c1-10(5-13-3-2-11-12-13)8(9(16)17)14-6(15)4-7(14)20(10,18)19;/h2-3,7-8H,4-5H2,1H3,(H,16,17);/q;+1/p-1/t7-,8+,10+;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides fragilis CcrA |
J Med Chem 47: 6556-68 (2004)
Article DOI: 10.1021/jm049680x
BindingDB Entry DOI: 10.7270/Q2542N22 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data