

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163650 (1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of rat eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of rat eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163661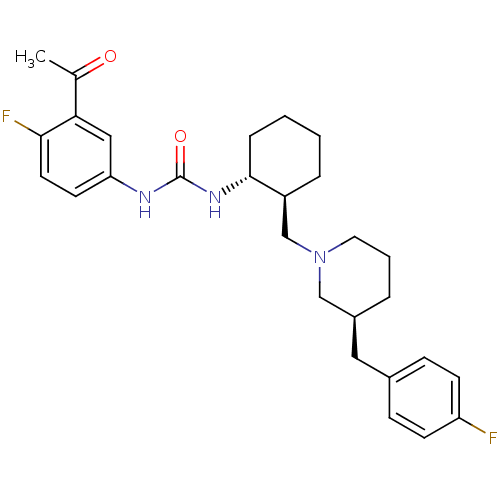 (1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410314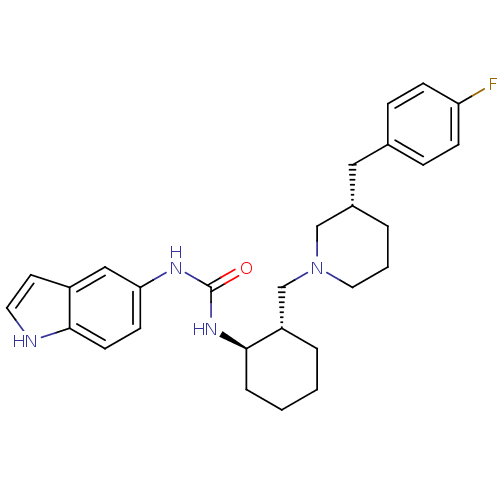 (CHEMBL2113074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of Cynomolgus monkey eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163678 (1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410322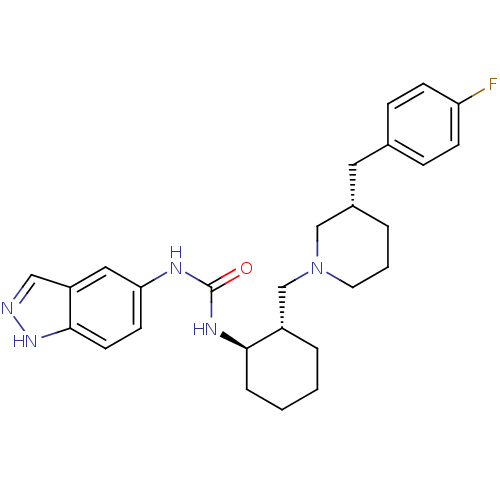 (CHEMBL2113077) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163662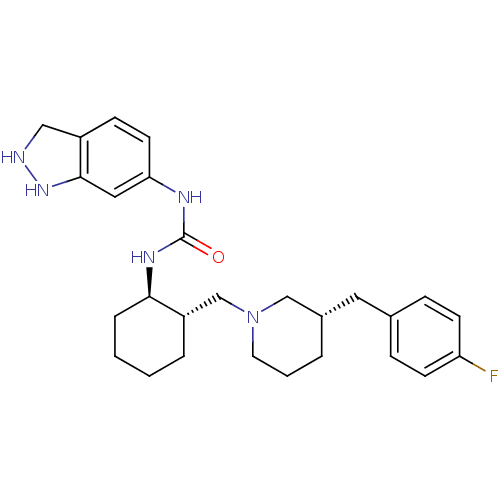 (1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163660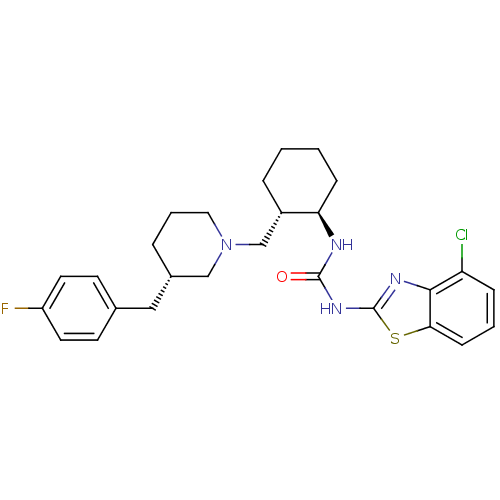 (1-(4-Chloro-benzothiazol-2-yl)-3-{(1R,2S)-2-[(S)-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163656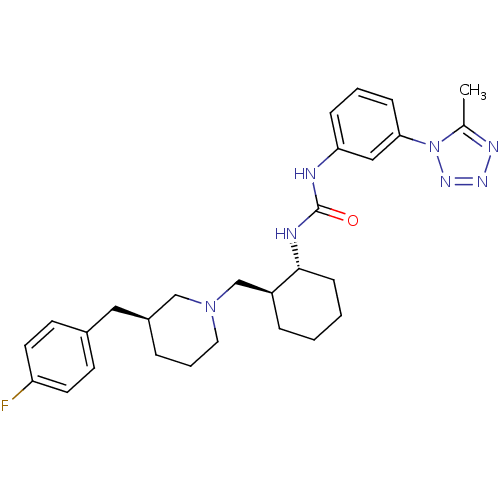 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163635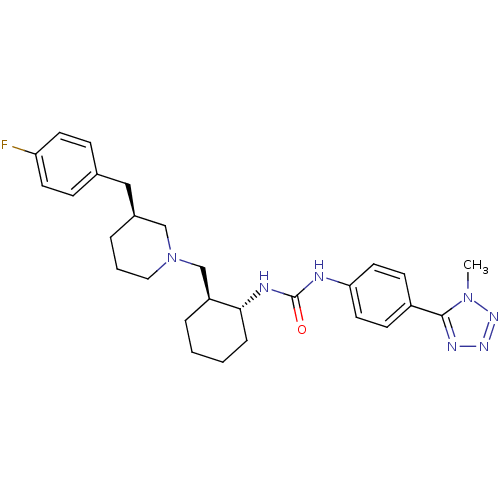 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410322 (CHEMBL2113077) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163645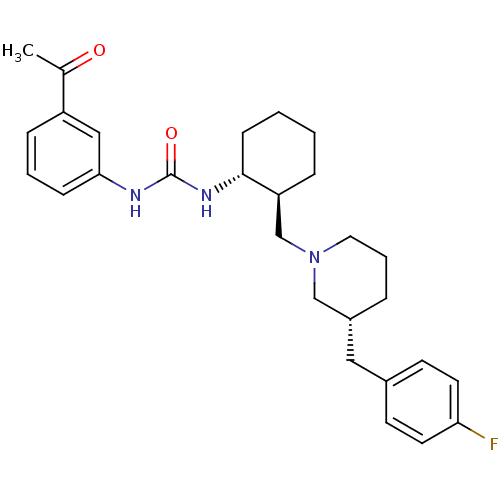 (1-(3-Acetyl-phenyl)-3-{(1R,2S)-2-[(R)-3-(4-fluoro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.364 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410314 (CHEMBL2113074) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163635 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163662 (1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163652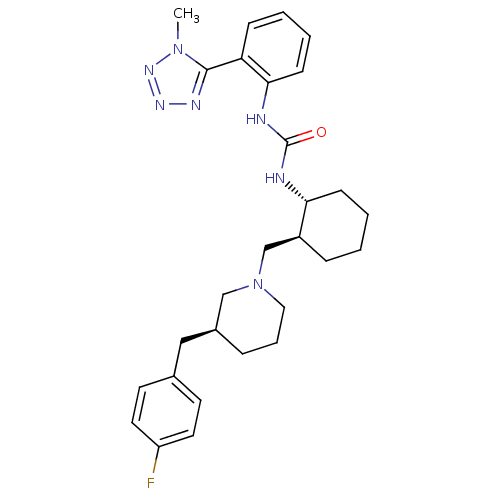 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50117461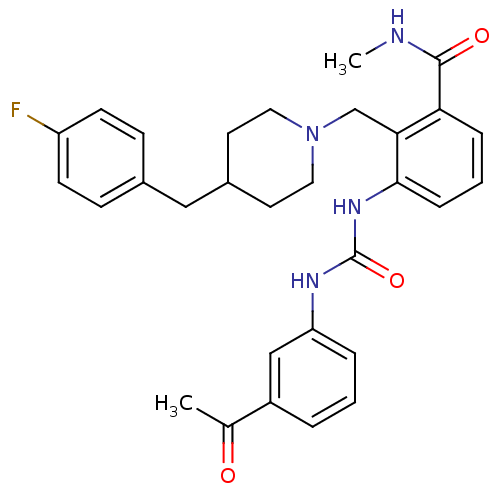 (3-[3-(3-Acetyl-phenyl)-ureido]-2-[4-(4-fluoro-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163678 (1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163634 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of calcium mobilization in human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163632 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163677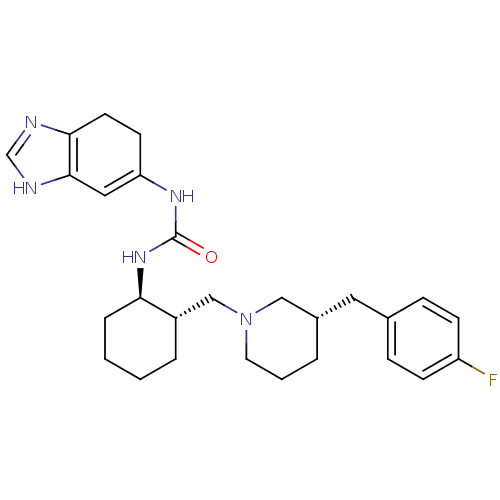 (1-(3a,4-Dihydro-1H-benzoimidazol-5-yl)-3-{(1R,2S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163682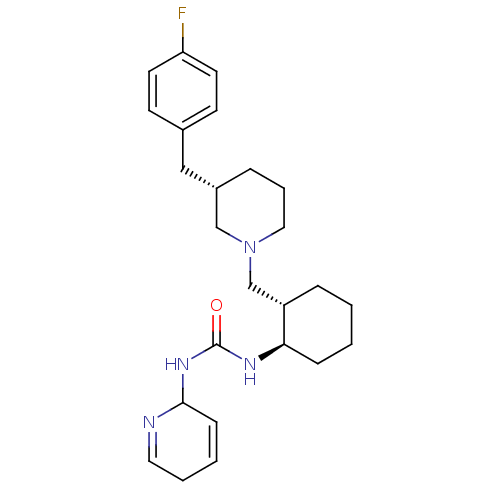 (1-(1,2-Dihydro-pyridin-2-yl)-3-{(1R,2S)-2-[(S)-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163650 (1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410321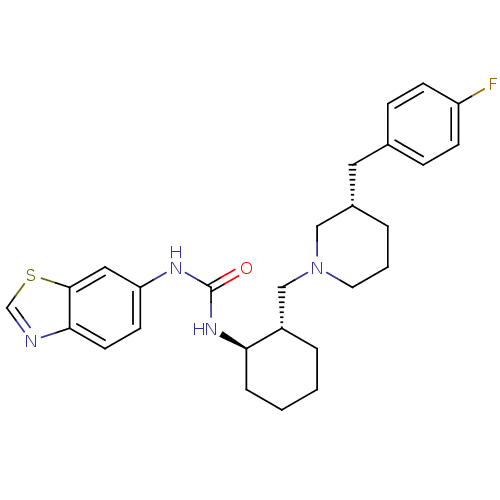 (CHEMBL2113076) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410323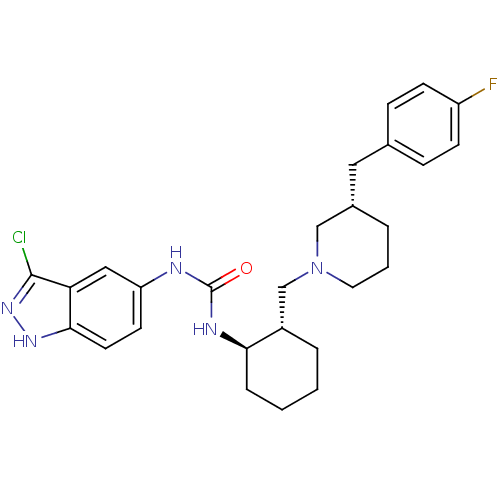 (CHEMBL2113079) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163656 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163670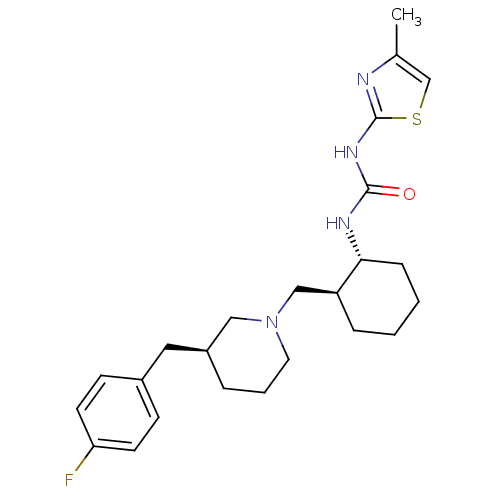 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163651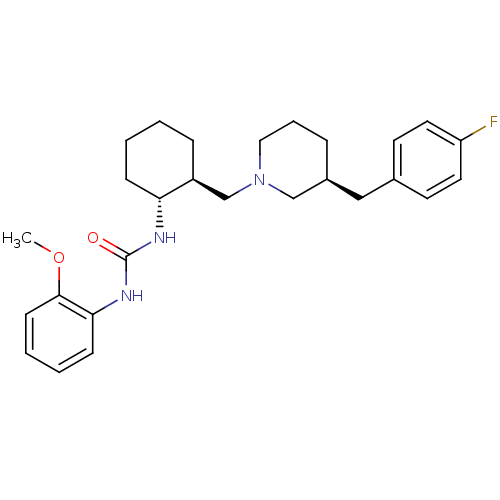 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163661 (1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163671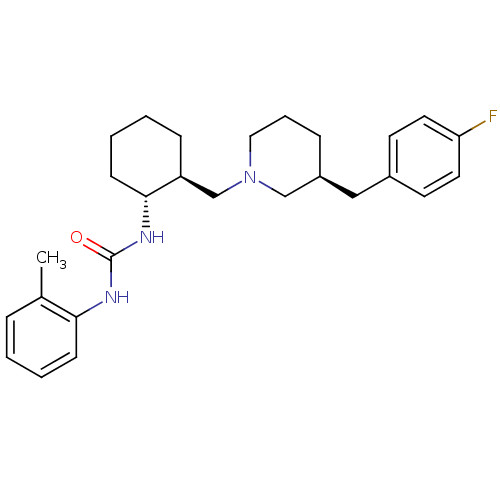 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163666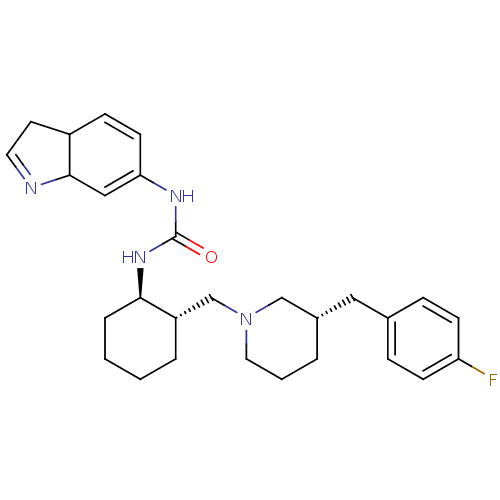 (1-(3a,7a-Dihydro-1H-indol-6-yl)-3-{(1R,2S)-2-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50410325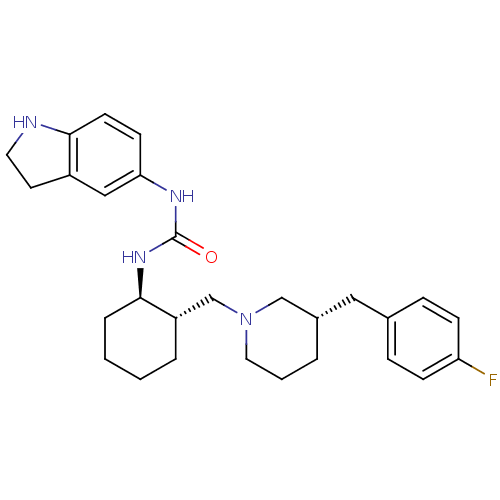 (CHEMBL2113080) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163675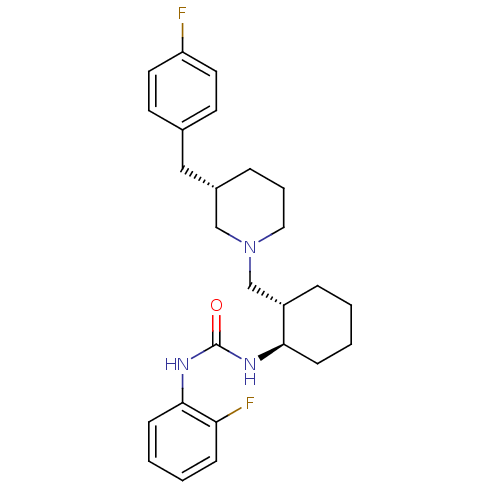 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163647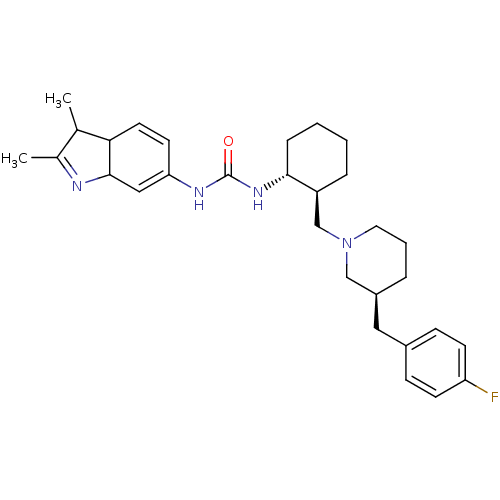 (1-(2,3-Dimethyl-3a,7a-dihydro-1H-indol-6-yl)-3-{(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163641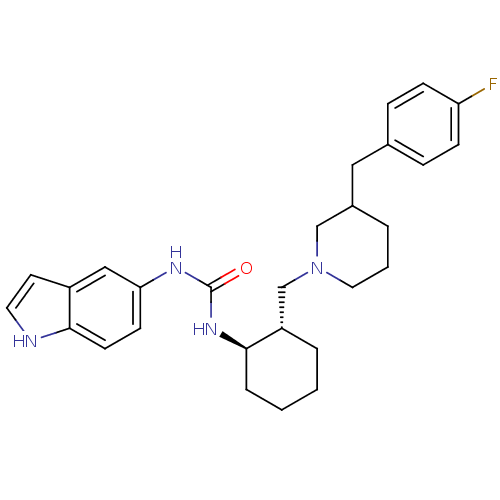 (1-{(1R,2S)-2-[3-(4-Fluoro-benzyl)-piperidin-1-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163637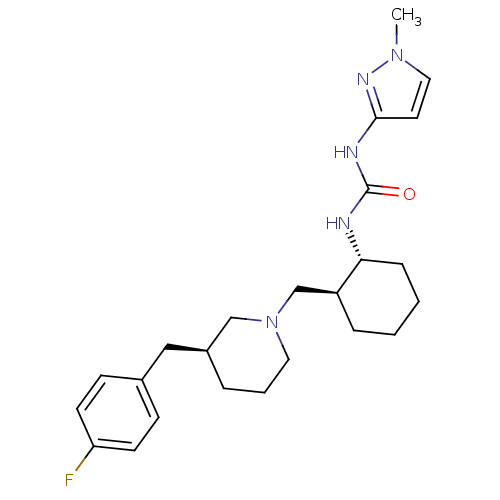 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163676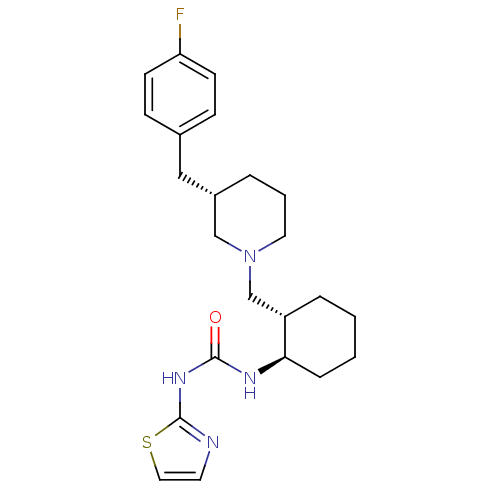 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163656 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of calcium mobilization in human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50163632 (1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |