

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50068350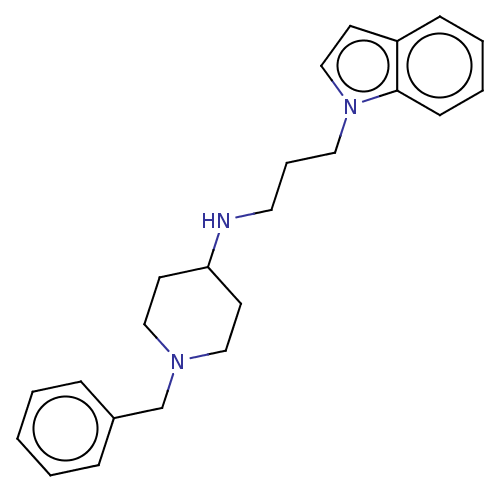 (CHEMBL3402303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068302 (CHEMBL3402305) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068306 (CHEMBL3402304) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068345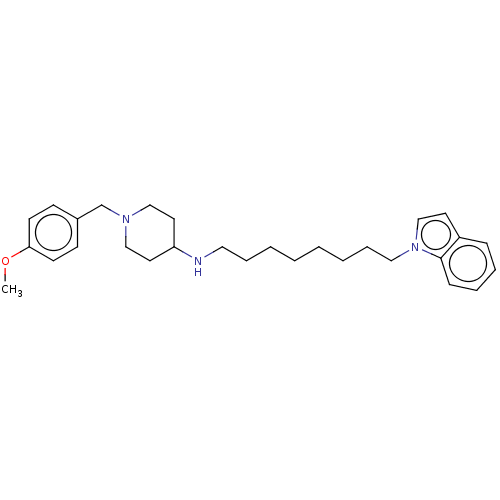 (CHEMBL3402314) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068344 (CHEMBL3402313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068348 (CHEMBL3402296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068296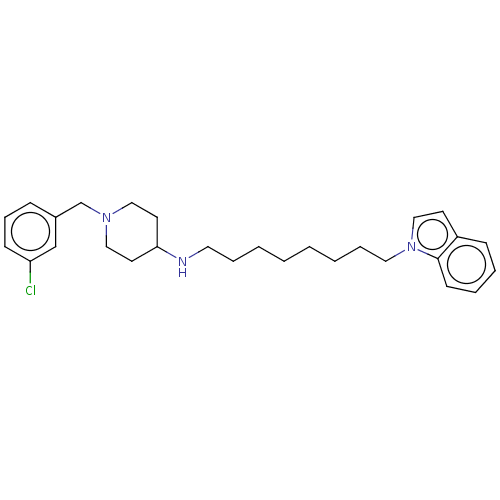 (CHEMBL3402310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068334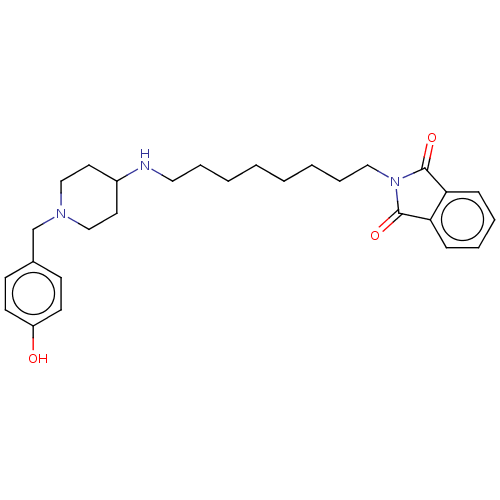 (CHEMBL3402299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068297 (CHEMBL3402309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068299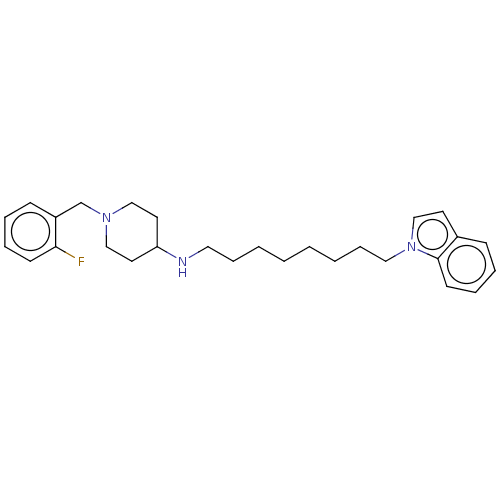 (CHEMBL3402308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068301 (CHEMBL3402306) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068342 (CHEMBL3402312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068349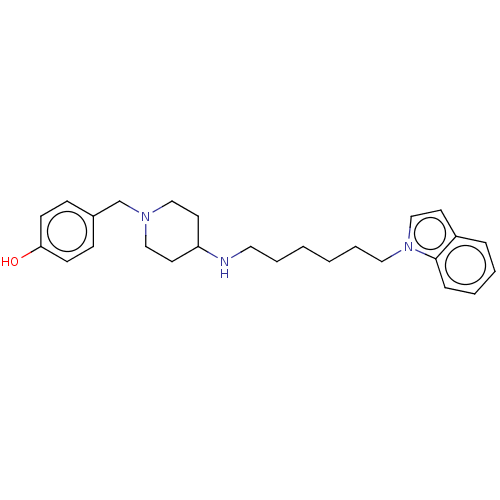 (CHEMBL3402311) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068306 (CHEMBL3402304) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068290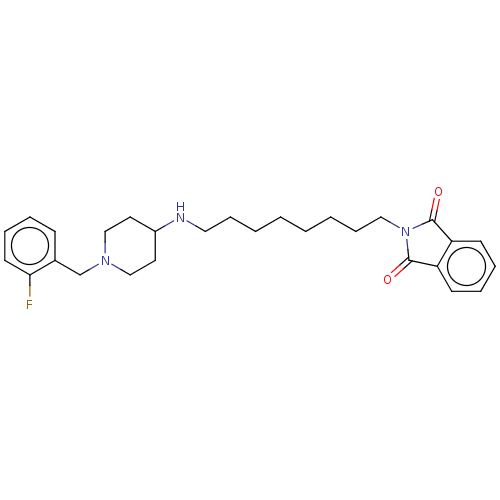 (CHEMBL3402293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068293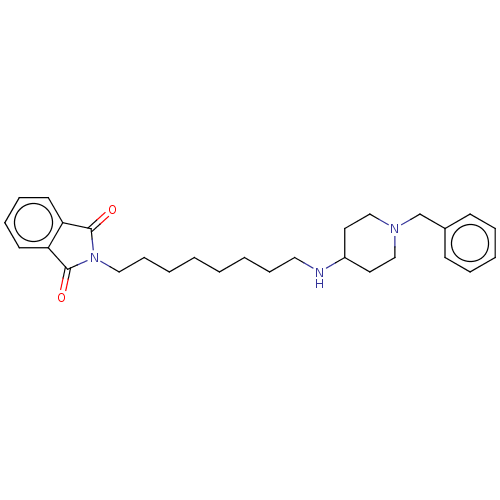 (CHEMBL3400171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068338 (CHEMBL3402298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068288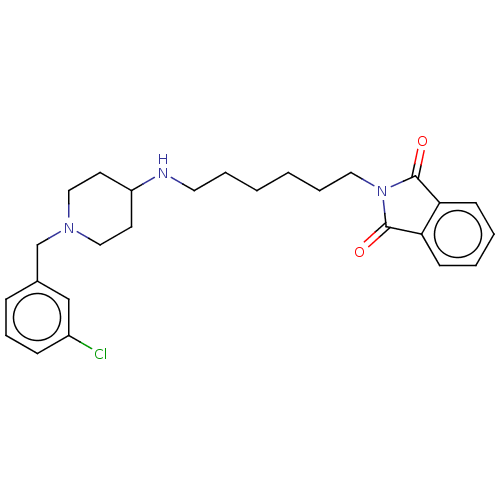 (CHEMBL3402295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068300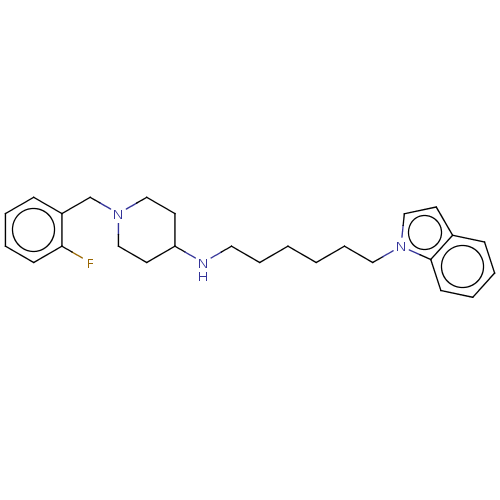 (CHEMBL3402307) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068291 (CHEMBL3402292) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068339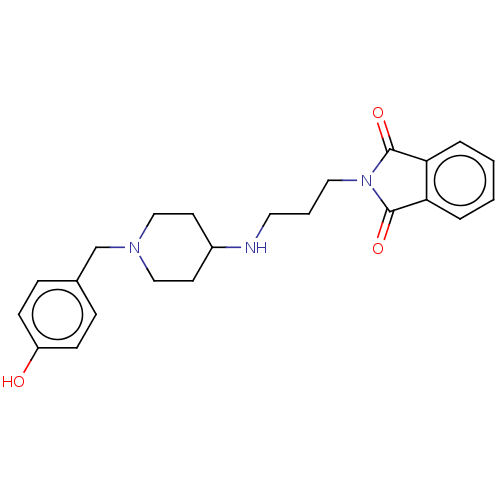 (CHEMBL3402297) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068289 (CHEMBL3402294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068319 (CHEMBL3402302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068346 (CHEMBL3402301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068295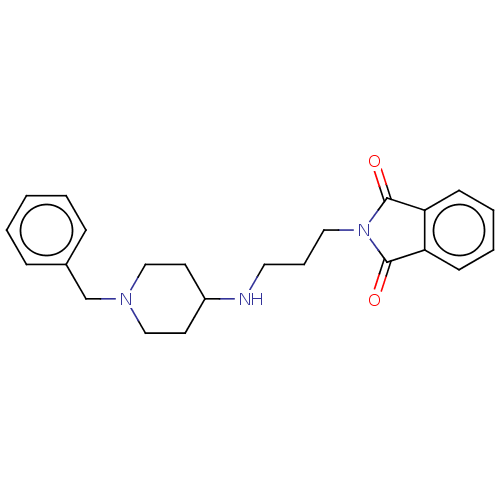 (CHEMBL3402288) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068339 (CHEMBL3402297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068292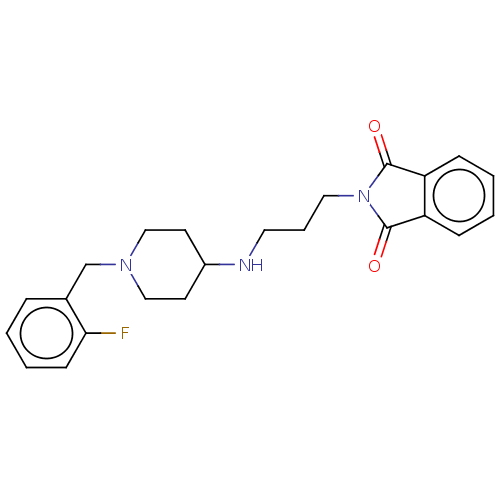 (CHEMBL3402291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068294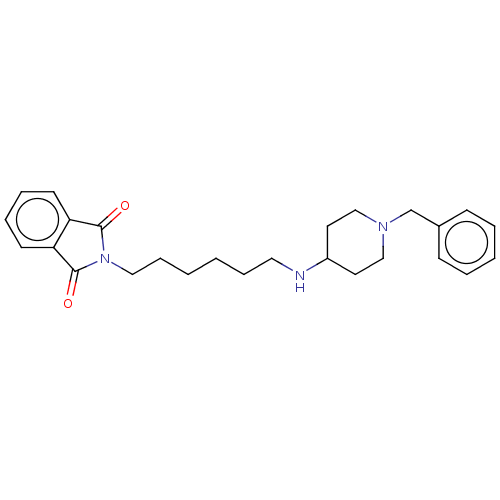 (CHEMBL3402290) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068302 (CHEMBL3402305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068293 (CHEMBL3400171) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068296 (CHEMBL3402310) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068292 (CHEMBL3402291) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068334 (CHEMBL3402299) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068347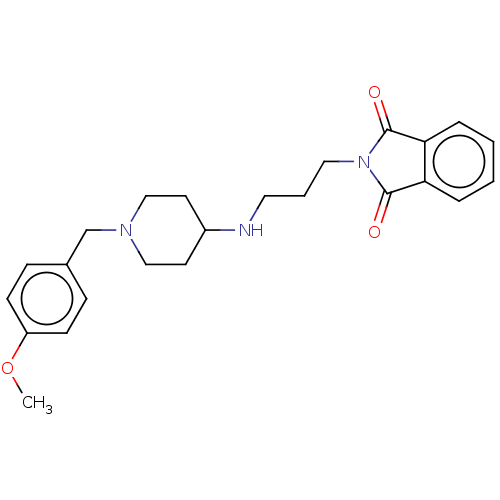 (CHEMBL3402300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068299 (CHEMBL3402308) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068338 (CHEMBL3402298) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068300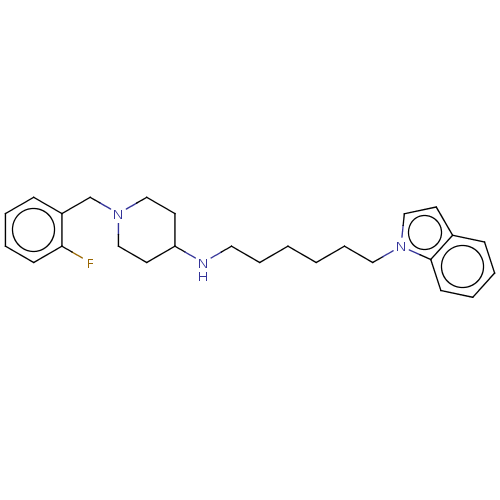 (CHEMBL3402307) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068319 (CHEMBL3402302) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068290 (CHEMBL3402293) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068294 (CHEMBL3402290) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50068295 (CHEMBL3402288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of horse serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068297 (CHEMBL3402309) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068288 (CHEMBL3402295) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068289 (CHEMBL3402294) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068341 (CHEMBL3402289) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50068301 (CHEMBL3402306) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins by Ellman method | Bioorg Med Chem 23: 2445-57 (2015) Article DOI: 10.1016/j.bmc.2015.03.051 BindingDB Entry DOI: 10.7270/Q2CZ38VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |