 Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50016895
Found 74 hits Enz. Inhib. hit(s) with all data for entry = 50016895 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174961
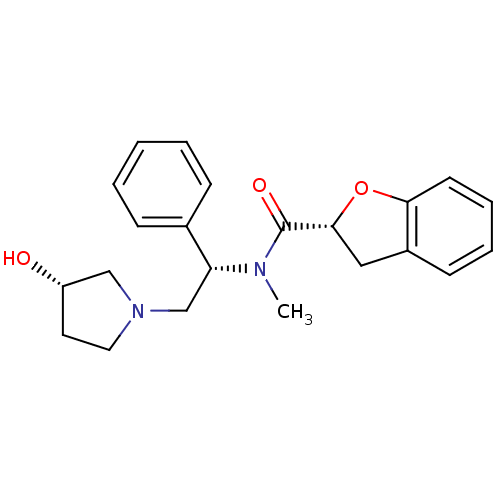
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1Cc2ccccc2O1 Show InChI InChI=1S/C22H26N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,18-19,21,25H,11-15H2,1H3/t18-,19+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174963
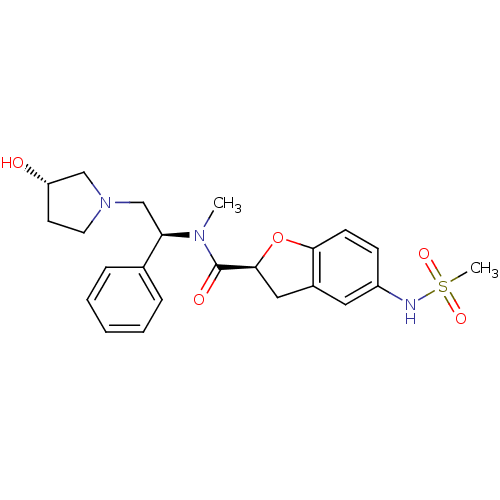
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1Cc2cc(NS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C23H29N3O5S/c1-25(20(16-6-4-3-5-7-16)15-26-11-10-19(27)14-26)23(28)22-13-17-12-18(24-32(2,29)30)8-9-21(17)31-22/h3-9,12,19-20,22,24,27H,10-11,13-15H2,1-2H3/t19-,20+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174967
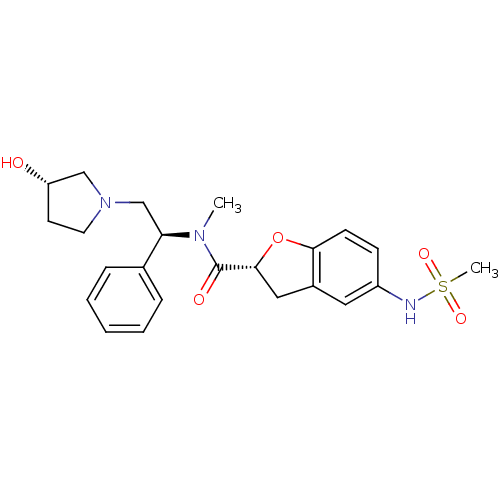
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1Cc2cc(NS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C23H29N3O5S/c1-25(20(16-6-4-3-5-7-16)15-26-11-10-19(27)14-26)23(28)22-13-17-12-18(24-32(2,29)30)8-9-21(17)31-22/h3-9,12,19-20,22,24,27H,10-11,13-15H2,1-2H3/t19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174957
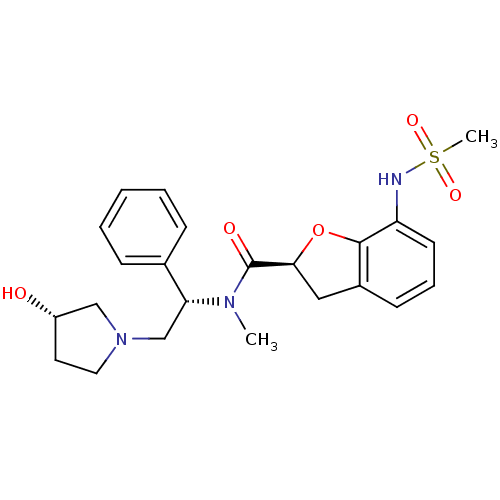
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1Cc2cccc(NS(C)(=O)=O)c2O1 Show InChI InChI=1S/C23H29N3O5S/c1-25(20(16-7-4-3-5-8-16)15-26-12-11-18(27)14-26)23(28)21-13-17-9-6-10-19(22(17)31-21)24-32(2,29)30/h3-10,18,20-21,24,27H,11-15H2,1-2H3/t18-,20+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174964
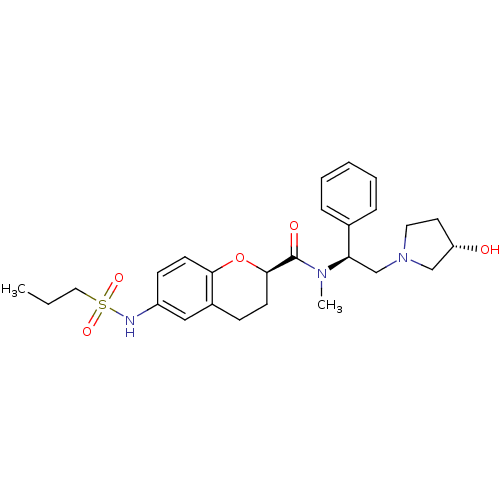
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)Nc1ccc2O[C@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-15-35(32,33)27-21-10-12-24-20(16-21)9-11-25(34-24)26(31)28(2)23(19-7-5-4-6-8-19)18-29-14-13-22(30)17-29/h4-8,10,12,16,22-23,25,27,30H,3,9,11,13-15,17-18H2,1-2H3/t22-,23+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174956
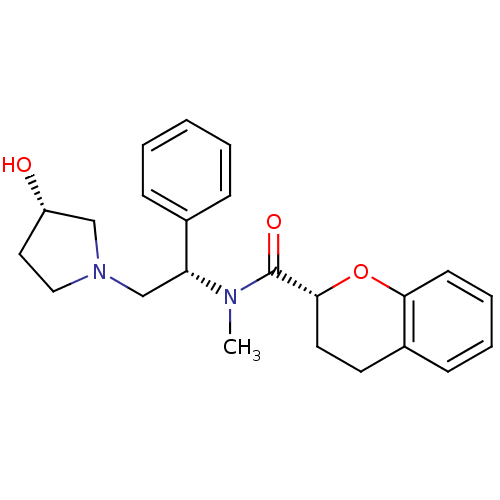
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1CCc2ccccc2O1 Show InChI InChI=1S/C23H28N2O3/c1-24(23(27)22-12-11-18-9-5-6-10-21(18)28-22)20(17-7-3-2-4-8-17)16-25-14-13-19(26)15-25/h2-10,19-20,22,26H,11-16H2,1H3/t19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174949

(CHEMBL371685 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(NS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C24H31N3O5S/c1-26(21(17-6-4-3-5-7-17)16-27-13-12-20(28)15-27)24(29)23-10-8-18-14-19(25-33(2,30)31)9-11-22(18)32-23/h3-7,9,11,14,20-21,23,25,28H,8,10,12-13,15-16H2,1-2H3/t20-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174955
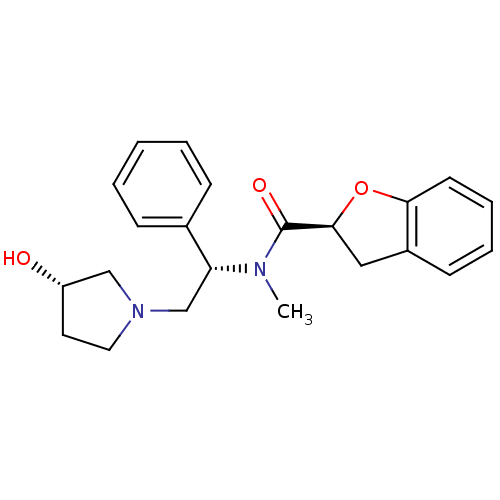
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1Cc2ccccc2O1 Show InChI InChI=1S/C22H26N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,18-19,21,25H,11-15H2,1H3/t18-,19+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174960
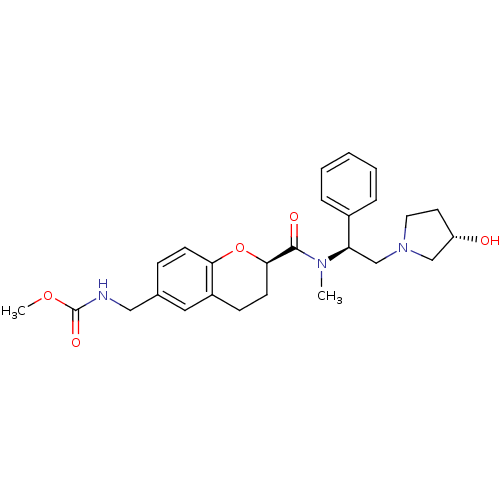
(CHEMBL197070 | methyl ((R)-2-(((S)-2-((S)-3-hydrox...)Show SMILES COC(=O)NCc1ccc2O[C@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H33N3O5/c1-28(22(19-6-4-3-5-7-19)17-29-13-12-21(30)16-29)25(31)24-11-9-20-14-18(8-10-23(20)34-24)15-27-26(32)33-2/h3-8,10,14,21-22,24,30H,9,11-13,15-17H2,1-2H3,(H,27,32)/t21-,22+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174950
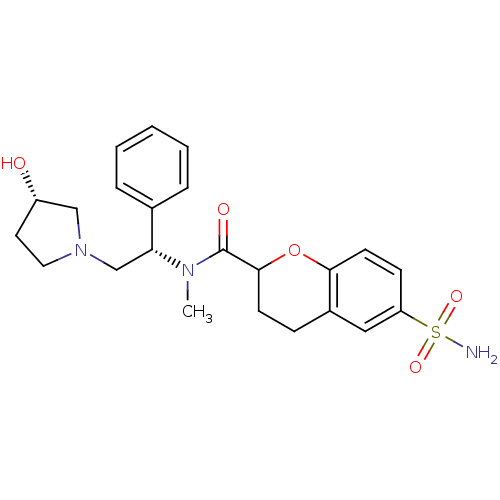
(6-Sulfamoyl-chroman-2-carboxylic acid [(S)-2-((S)-...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(ccc2O1)S(N)(=O)=O Show InChI InChI=1S/C23H29N3O5S/c1-25(20(16-5-3-2-4-6-16)15-26-12-11-18(27)14-26)23(28)22-9-7-17-13-19(32(24,29)30)8-10-21(17)31-22/h2-6,8,10,13,18,20,22,27H,7,9,11-12,14-15H2,1H3,(H2,24,29,30)/t18-,20+,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174944
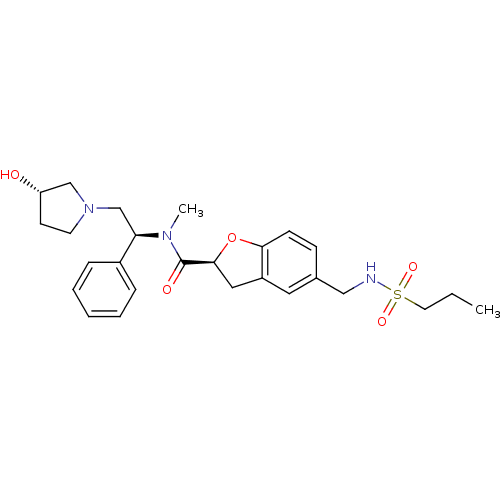
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)NCc1ccc2O[C@@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-13-35(32,33)27-16-19-9-10-24-21(14-19)15-25(34-24)26(31)28(2)23(20-7-5-4-6-8-20)18-29-12-11-22(30)17-29/h4-10,14,22-23,25,27,30H,3,11-13,15-18H2,1-2H3/t22-,23+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174945
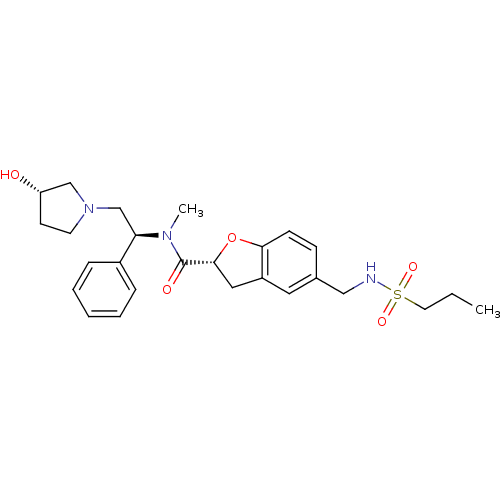
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)NCc1ccc2O[C@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-13-35(32,33)27-16-19-9-10-24-21(14-19)15-25(34-24)26(31)28(2)23(20-7-5-4-6-8-20)18-29-12-11-22(30)17-29/h4-10,14,22-23,25,27,30H,3,11-13,15-18H2,1-2H3/t22-,23+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174948
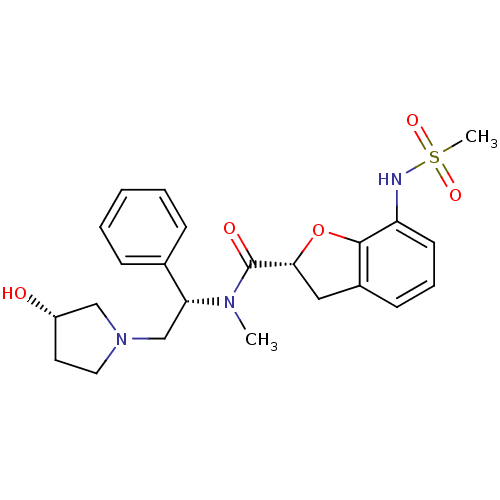
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1Cc2cccc(NS(C)(=O)=O)c2O1 Show InChI InChI=1S/C23H29N3O5S/c1-25(20(16-7-4-3-5-8-16)15-26-12-11-18(27)14-26)23(28)21-13-17-9-6-10-19(22(17)31-21)24-32(2,29)30/h3-10,18,20-21,24,27H,11-15H2,1-2H3/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174968
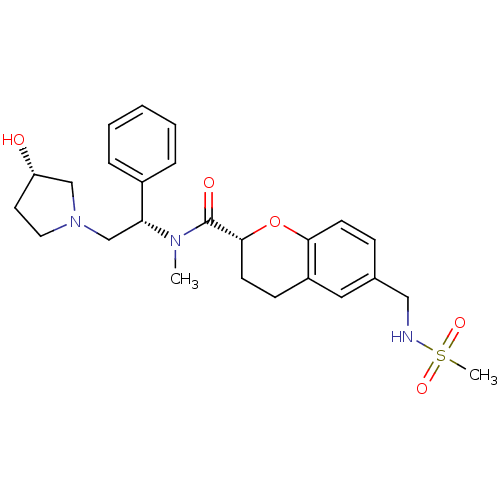
((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1CCc2cc(CNS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C25H33N3O5S/c1-27(22(19-6-4-3-5-7-19)17-28-13-12-21(29)16-28)25(30)24-11-9-20-14-18(8-10-23(20)33-24)15-26-34(2,31)32/h3-8,10,14,21-22,24,26,29H,9,11-13,15-17H2,1-2H3/t21-,22+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174962
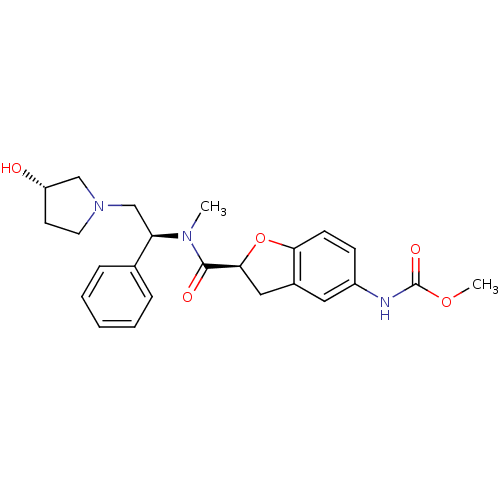
(CHEMBL197126 | methyl (S)-2-(((S)-2-((S)-3-hydroxy...)Show SMILES COC(=O)Nc1ccc2O[C@@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C24H29N3O5/c1-26(20(16-6-4-3-5-7-16)15-27-11-10-19(28)14-27)23(29)22-13-17-12-18(25-24(30)31-2)8-9-21(17)32-22/h3-9,12,19-20,22,28H,10-11,13-15H2,1-2H3,(H,25,30)/t19-,20+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174952
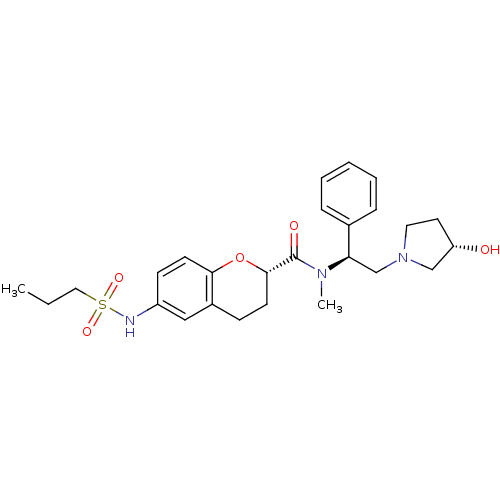
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)Nc1ccc2O[C@@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-15-35(32,33)27-21-10-12-24-20(16-21)9-11-25(34-24)26(31)28(2)23(19-7-5-4-6-8-19)18-29-14-13-22(30)17-29/h4-8,10,12,16,22-23,25,27,30H,3,9,11,13-15,17-18H2,1-2H3/t22-,23+,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174947
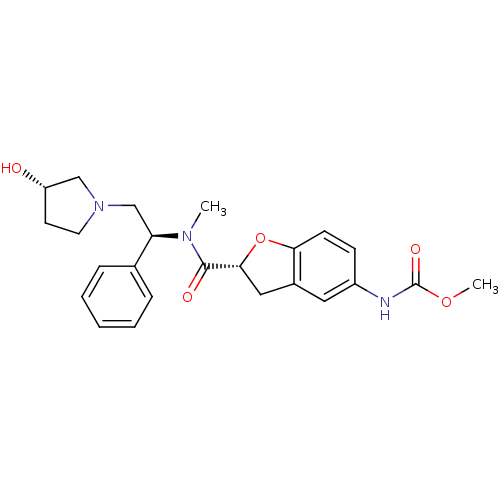
(CHEMBL198487 | methyl (R)-2-(((S)-2-((S)-3-hydroxy...)Show SMILES COC(=O)Nc1ccc2O[C@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C24H29N3O5/c1-26(20(16-6-4-3-5-7-16)15-27-11-10-19(28)14-27)23(29)22-13-17-12-18(25-24(30)31-2)8-9-21(17)32-22/h3-9,12,19-20,22,28H,10-11,13-15H2,1-2H3,(H,25,30)/t19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174943
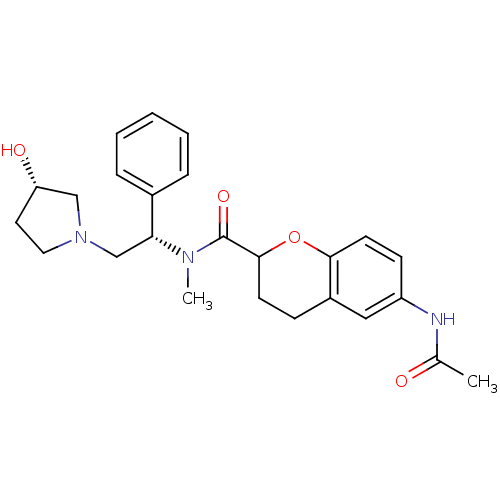
(6-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C25H31N3O4/c1-17(29)26-20-9-11-23-19(14-20)8-10-24(32-23)25(31)27(2)22(18-6-4-3-5-7-18)16-28-13-12-21(30)15-28/h3-7,9,11,14,21-22,24,30H,8,10,12-13,15-16H2,1-2H3,(H,26,29)/t21-,22+,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174966
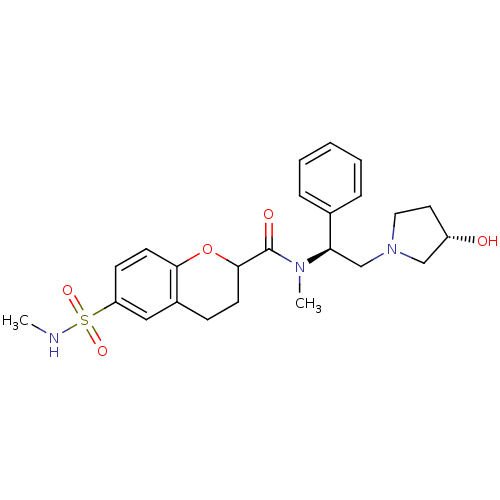
(6-Methylsulfamoyl-chroman-2-carboxylic acid [(S)-2...)Show SMILES CNS(=O)(=O)c1ccc2OC(CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C24H31N3O5S/c1-25-33(30,31)20-9-11-22-18(14-20)8-10-23(32-22)24(29)26(2)21(17-6-4-3-5-7-17)16-27-13-12-19(28)15-27/h3-7,9,11,14,19,21,23,25,28H,8,10,12-13,15-16H2,1-2H3/t19-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174965
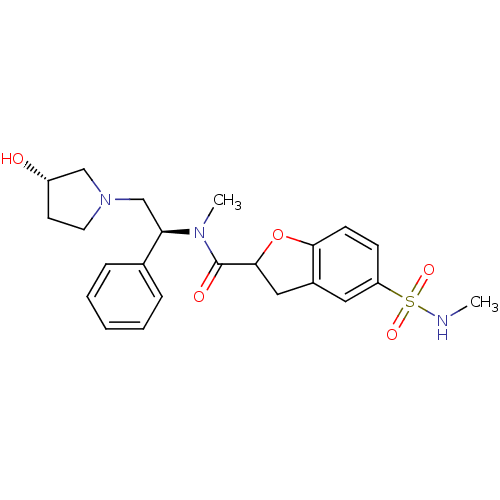
(5-Methylsulfamoyl-2,3-dihydro-benzofuran-2-carboxy...)Show SMILES CNS(=O)(=O)c1ccc2OC(Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C23H29N3O5S/c1-24-32(29,30)19-8-9-21-17(12-19)13-22(31-21)23(28)25(2)20(16-6-4-3-5-7-16)15-26-11-10-18(27)14-26/h3-9,12,18,20,22,24,27H,10-11,13-15H2,1-2H3/t18-,20+,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174958
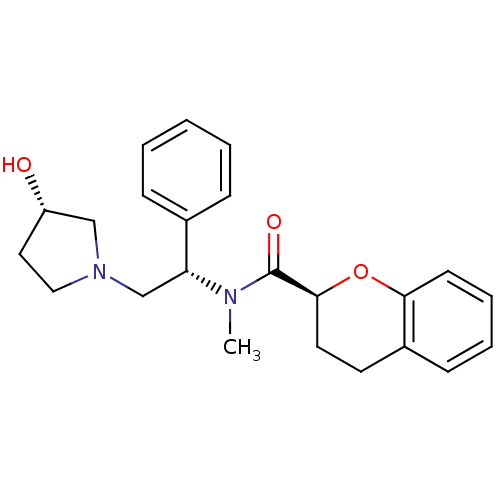
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1CCc2ccccc2O1 Show InChI InChI=1S/C23H28N2O3/c1-24(23(27)22-12-11-18-9-5-6-10-21(18)28-22)20(17-7-3-2-4-8-17)16-25-14-13-19(26)15-25/h2-10,19-20,22,26H,11-16H2,1H3/t19-,20+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174946
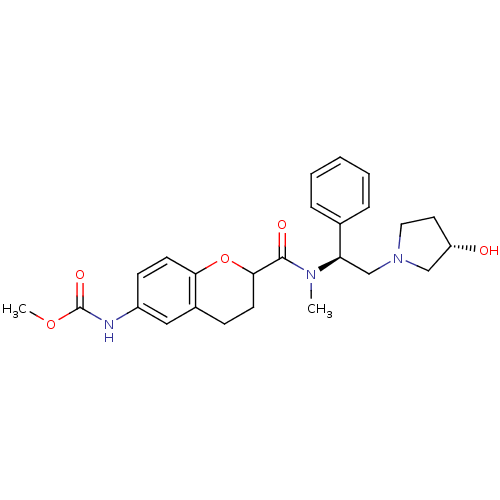
(CHEMBL197293 | methyl 2-(((S)-2-((S)-3-hydroxypyrr...)Show SMILES COC(=O)Nc1ccc2OC(CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C25H31N3O5/c1-27(21(17-6-4-3-5-7-17)16-28-13-12-20(29)15-28)24(30)23-10-8-18-14-19(26-25(31)32-2)9-11-22(18)33-23/h3-7,9,11,14,20-21,23,29H,8,10,12-13,15-16H2,1-2H3,(H,26,31)/t20-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174953
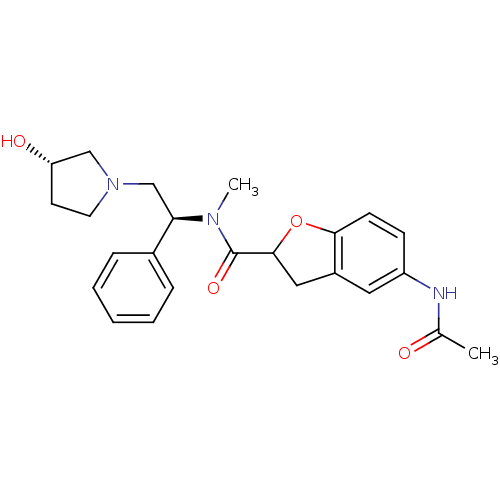
(5-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1Cc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C24H29N3O4/c1-16(28)25-19-8-9-22-18(12-19)13-23(31-22)24(30)26(2)21(17-6-4-3-5-7-17)15-27-11-10-20(29)14-27/h3-9,12,20-21,23,29H,10-11,13-15H2,1-2H3,(H,25,28)/t20-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174954
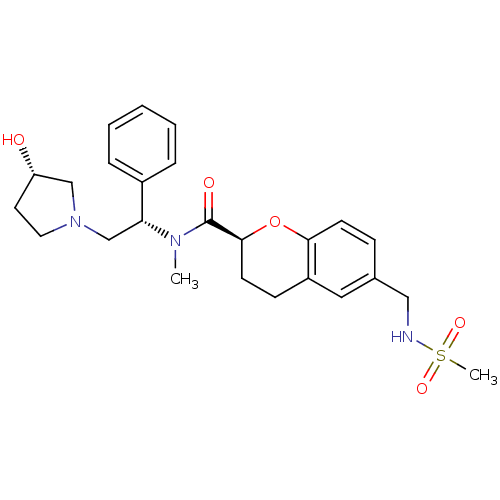
((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1CCc2cc(CNS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C25H33N3O5S/c1-27(22(19-6-4-3-5-7-19)17-28-13-12-21(29)16-28)25(30)24-11-9-20-14-18(8-10-23(20)33-24)15-26-34(2,31)32/h3-8,10,14,21-22,24,26,29H,9,11-13,15-17H2,1-2H3/t21-,22+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174959
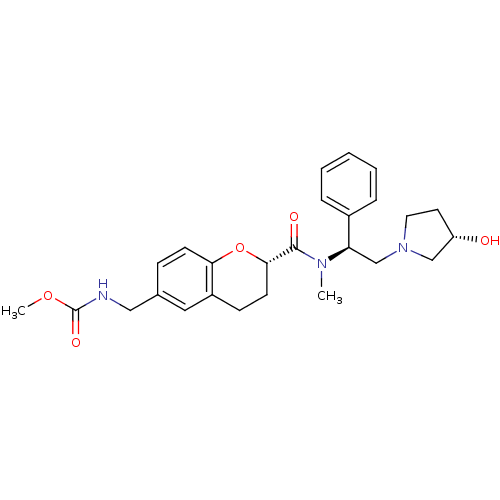
(CHEMBL196813 | methyl ((S)-2-(((S)-2-((S)-3-hydrox...)Show SMILES COC(=O)NCc1ccc2O[C@@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H33N3O5/c1-28(22(19-6-4-3-5-7-19)17-29-13-12-21(30)16-29)25(31)24-11-9-20-14-18(8-10-23(20)34-24)15-27-26(32)33-2/h3-8,10,14,21-22,24,30H,9,11-13,15-17H2,1-2H3,(H,27,32)/t21-,22+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50174951
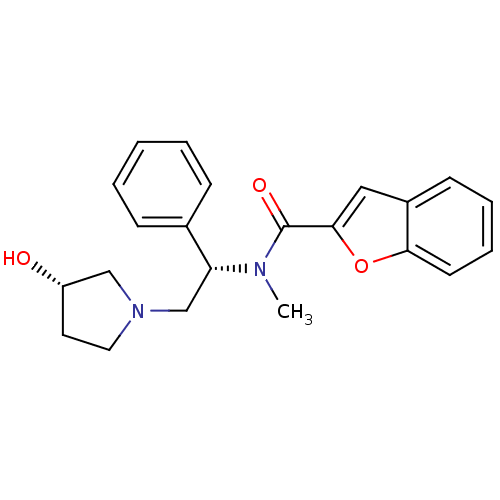
(CHEMBL200478 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C22H24N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,13,18-19,25H,11-12,14-15H2,1H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at kappa opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174943

(6-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C25H31N3O4/c1-17(29)26-20-9-11-23-19(14-20)8-10-24(32-23)25(31)27(2)22(18-6-4-3-5-7-18)16-28-13-12-21(30)15-28/h3-7,9,11,14,21-22,24,30H,8,10,12-13,15-16H2,1-2H3,(H,26,29)/t21-,22+,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174946

(CHEMBL197293 | methyl 2-(((S)-2-((S)-3-hydroxypyrr...)Show SMILES COC(=O)Nc1ccc2OC(CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C25H31N3O5/c1-27(21(17-6-4-3-5-7-17)16-28-13-12-20(29)15-28)24(30)23-10-8-18-14-19(26-25(31)32-2)9-11-22(18)33-23/h3-7,9,11,14,20-21,23,29H,8,10,12-13,15-16H2,1-2H3,(H,26,31)/t20-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174947

(CHEMBL198487 | methyl (R)-2-(((S)-2-((S)-3-hydroxy...)Show SMILES COC(=O)Nc1ccc2O[C@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C24H29N3O5/c1-26(20(16-6-4-3-5-7-16)15-27-11-10-19(28)14-27)23(29)22-13-17-12-18(25-24(30)31-2)8-9-21(17)32-22/h3-9,12,19-20,22,28H,10-11,13-15H2,1-2H3,(H,25,30)/t19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174951

(CHEMBL200478 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C22H24N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,13,18-19,25H,11-12,14-15H2,1H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174953

(5-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1Cc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C24H29N3O4/c1-16(28)25-19-8-9-22-18(12-19)13-23(31-22)24(30)26(2)21(17-6-4-3-5-7-17)15-27-11-10-20(29)14-27/h3-9,12,20-21,23,29H,10-11,13-15H2,1-2H3,(H,25,28)/t20-,21+,23?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50174956

((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1CCc2ccccc2O1 Show InChI InChI=1S/C23H28N2O3/c1-24(23(27)22-12-11-18-9-5-6-10-21(18)28-22)20(17-7-3-2-4-8-17)16-25-14-13-19(26)15-25/h2-10,19-20,22,26H,11-16H2,1H3/t19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at delta opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174949

(CHEMBL371685 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(NS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C24H31N3O5S/c1-26(21(17-6-4-3-5-7-17)16-27-13-12-20(28)15-27)24(29)23-10-8-18-14-19(25-33(2,30)31)9-11-22(18)32-23/h3-7,9,11,14,20-21,23,25,28H,8,10,12-13,15-16H2,1-2H3/t20-,21+,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174951

(CHEMBL200478 | N-((S)-2-((S)-3-hydroxypyrrolidin-1...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)c1cc2ccccc2o1 Show InChI InChI=1S/C22H24N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,13,18-19,25H,11-12,14-15H2,1H3/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174954

((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1CCc2cc(CNS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C25H33N3O5S/c1-27(22(19-6-4-3-5-7-19)17-28-13-12-21(29)16-28)25(30)24-11-9-20-14-18(8-10-23(20)33-24)15-26-34(2,31)32/h3-8,10,14,21-22,24,26,29H,9,11-13,15-17H2,1-2H3/t21-,22+,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174953

(5-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1Cc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C24H29N3O4/c1-16(28)25-19-8-9-22-18(12-19)13-23(31-22)24(30)26(2)21(17-6-4-3-5-7-17)15-27-11-10-20(29)14-27/h3-9,12,20-21,23,29H,10-11,13-15H2,1-2H3,(H,25,28)/t20-,21+,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174956

((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1CCc2ccccc2O1 Show InChI InChI=1S/C23H28N2O3/c1-24(23(27)22-12-11-18-9-5-6-10-21(18)28-22)20(17-7-3-2-4-8-17)16-25-14-13-19(26)15-25/h2-10,19-20,22,26H,11-16H2,1H3/t19-,20+,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174945

((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)NCc1ccc2O[C@H](Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-13-35(32,33)27-16-19-9-10-24-21(14-19)15-25(34-24)26(31)28(2)23(20-7-5-4-6-8-20)18-29-12-11-22(30)17-29/h4-10,14,22-23,25,27,30H,3,11-13,15-18H2,1-2H3/t22-,23+,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174959

(CHEMBL196813 | methyl ((S)-2-(((S)-2-((S)-3-hydrox...)Show SMILES COC(=O)NCc1ccc2O[C@@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H33N3O5/c1-28(22(19-6-4-3-5-7-19)17-29-13-12-21(30)16-29)25(31)24-11-9-20-14-18(8-10-23(20)34-24)15-27-26(32)33-2/h3-8,10,14,21-22,24,30H,9,11-13,15-17H2,1-2H3,(H,27,32)/t21-,22+,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174958

((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1CCc2ccccc2O1 Show InChI InChI=1S/C23H28N2O3/c1-24(23(27)22-12-11-18-9-5-6-10-21(18)28-22)20(17-7-3-2-4-8-17)16-25-14-13-19(26)15-25/h2-10,19-20,22,26H,11-16H2,1H3/t19-,20+,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174960

(CHEMBL197070 | methyl ((R)-2-(((S)-2-((S)-3-hydrox...)Show SMILES COC(=O)NCc1ccc2O[C@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H33N3O5/c1-28(22(19-6-4-3-5-7-19)17-29-13-12-21(30)16-29)25(31)24-11-9-20-14-18(8-10-23(20)34-24)15-27-26(32)33-2/h3-8,10,14,21-22,24,30H,9,11-13,15-17H2,1-2H3,(H,27,32)/t21-,22+,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174961

((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1Cc2ccccc2O1 Show InChI InChI=1S/C22H26N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,18-19,21,25H,11-15H2,1H3/t18-,19+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174946

(CHEMBL197293 | methyl 2-(((S)-2-((S)-3-hydroxypyrr...)Show SMILES COC(=O)Nc1ccc2OC(CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C25H31N3O5/c1-27(21(17-6-4-3-5-7-17)16-28-13-12-20(29)15-28)24(30)23-10-8-18-14-19(26-25(31)32-2)9-11-22(18)33-23/h3-7,9,11,14,20-21,23,29H,8,10,12-13,15-16H2,1-2H3,(H,26,31)/t20-,21+,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174952

((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CCCS(=O)(=O)Nc1ccc2O[C@@H](CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C26H35N3O5S/c1-3-15-35(32,33)27-21-10-12-24-20(16-21)9-11-25(34-24)26(31)28(2)23(19-7-5-4-6-8-19)18-29-14-13-22(30)17-29/h4-8,10,12,16,22-23,25,27,30H,3,9,11,13-15,17-18H2,1-2H3/t22-,23+,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174955

((S)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@@H]1Cc2ccccc2O1 Show InChI InChI=1S/C22H26N2O3/c1-23(22(26)21-13-17-9-5-6-10-20(17)27-21)19(16-7-3-2-4-8-16)15-24-12-11-18(25)14-24/h2-10,18-19,21,25H,11-15H2,1H3/t18-,19+,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174966

(6-Methylsulfamoyl-chroman-2-carboxylic acid [(S)-2...)Show SMILES CNS(=O)(=O)c1ccc2OC(CCc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C24H31N3O5S/c1-25-33(30,31)20-9-11-22-18(14-20)8-10-23(32-22)24(29)26(2)21(17-6-4-3-5-7-17)16-27-13-12-19(28)15-27/h3-7,9,11,14,19,21,23,25,28H,8,10,12-13,15-16H2,1-2H3/t19-,21+,23?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174965

(5-Methylsulfamoyl-2,3-dihydro-benzofuran-2-carboxy...)Show SMILES CNS(=O)(=O)c1ccc2OC(Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C23H29N3O5S/c1-24-32(29,30)19-8-9-21-17(12-19)13-22(31-21)23(28)25(2)20(16-6-4-3-5-7-16)15-26-11-10-18(27)14-26/h3-9,12,18,20,22,24,27H,10-11,13-15H2,1-2H3/t18-,20+,22?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174968

((R)-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl)-1-phen...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)[C@H]1CCc2cc(CNS(C)(=O)=O)ccc2O1 Show InChI InChI=1S/C25H33N3O5S/c1-27(22(19-6-4-3-5-7-19)17-28-13-12-21(29)16-28)25(30)24-11-9-20-14-18(8-10-23(20)33-24)15-26-34(2,31)32/h3-8,10,14,21-22,24,26,29H,9,11-13,15-17H2,1-2H3/t21-,22+,24+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50174943

(6-acetamido-N-((S)-2-((S)-3-hydroxypyrrolidin-1-yl...)Show SMILES CN([C@H](CN1CC[C@H](O)C1)c1ccccc1)C(=O)C1CCc2cc(NC(C)=O)ccc2O1 Show InChI InChI=1S/C25H31N3O4/c1-17(29)26-20-9-11-23-19(14-20)8-10-24(32-23)25(31)27(2)22(18-6-4-3-5-7-18)16-28-13-12-21(30)15-28/h3-7,9,11,14,21-22,24,30H,8,10,12-13,15-16H2,1-2H3,(H,26,29)/t21-,22+,24?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonistic activity at mu opioid receptor |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50174965

(5-Methylsulfamoyl-2,3-dihydro-benzofuran-2-carboxy...)Show SMILES CNS(=O)(=O)c1ccc2OC(Cc2c1)C(=O)N(C)[C@H](CN1CC[C@H](O)C1)c1ccccc1 Show InChI InChI=1S/C23H29N3O5S/c1-24-32(29,30)19-8-9-21-17(12-19)13-22(31-21)23(28)25(2)20(16-6-4-3-5-7-16)15-26-11-10-18(27)14-26/h3-9,12,18,20,22,24,27H,10-11,13-15H2,1-2H3/t18-,20+,22?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against CYP2D6 |
Bioorg Med Chem Lett 15: 5114-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.094
BindingDB Entry DOI: 10.7270/Q23F4P6H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data