

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078672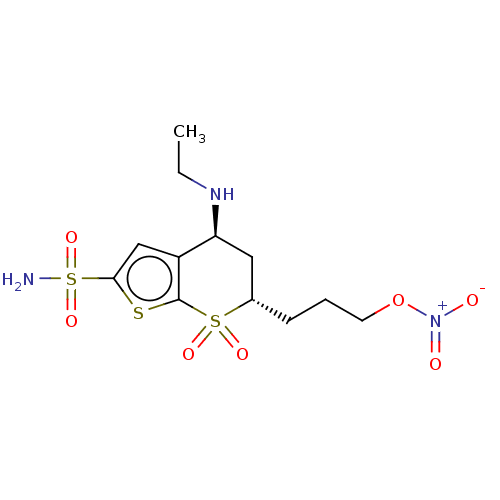 (CHEMBL3415379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078643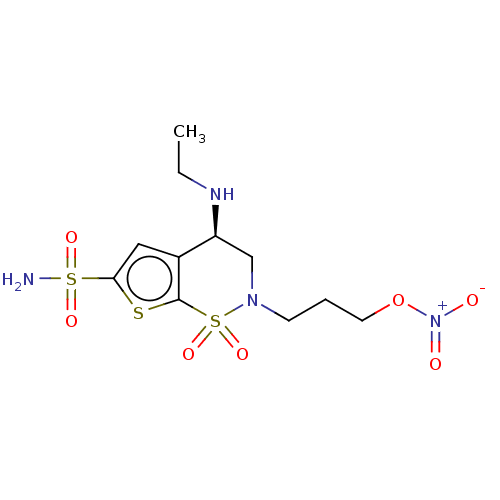 (CHEMBL3415383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078671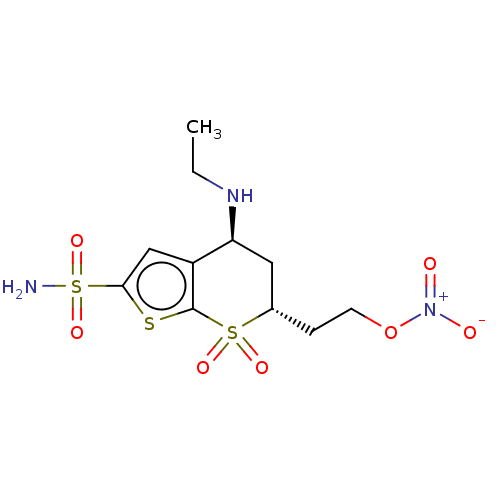 (CHEMBL3415380) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078644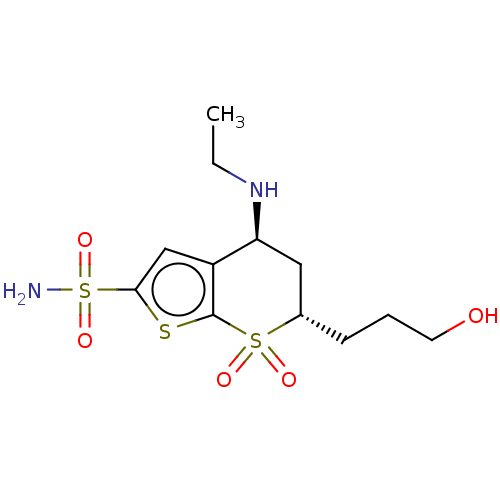 (CHEMBL3415382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078670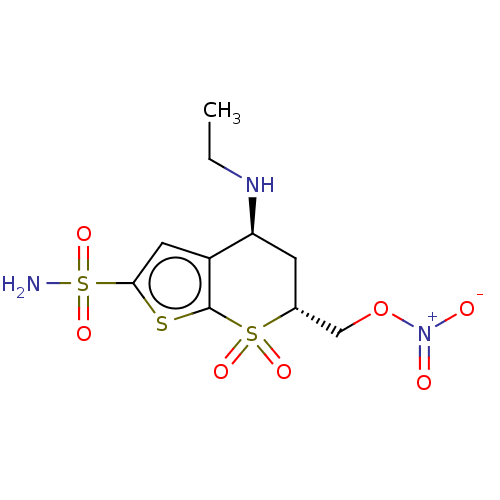 (CHEMBL3415381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078642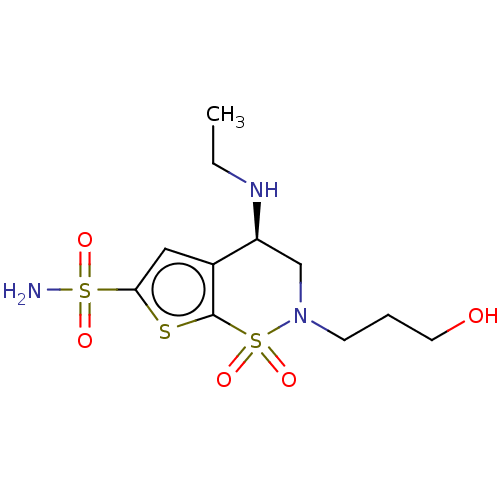 (CHEMBL3415384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 assessed as reduction in enzyme activity incubated for 30 mins by fluorescence polarization assay | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078671 (CHEMBL3415380) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078672 (CHEMBL3415379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078670 (CHEMBL3415381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078644 (CHEMBL3415382) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078642 (CHEMBL3415384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.136 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50078643 (CHEMBL3415383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human carbonic anhydrase 2 incubated for 1 hr using labeled BODIPY558/568-acetazolamide as tracer by fluorescence polarization ti... | J Med Chem 58: 2821-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00043 BindingDB Entry DOI: 10.7270/Q2QF8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||