

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084436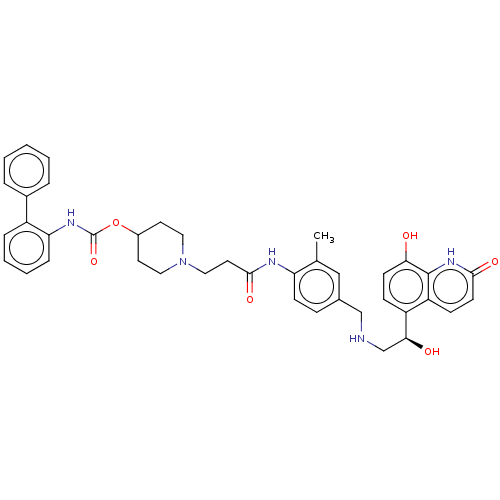 (CHEMBL3426693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084443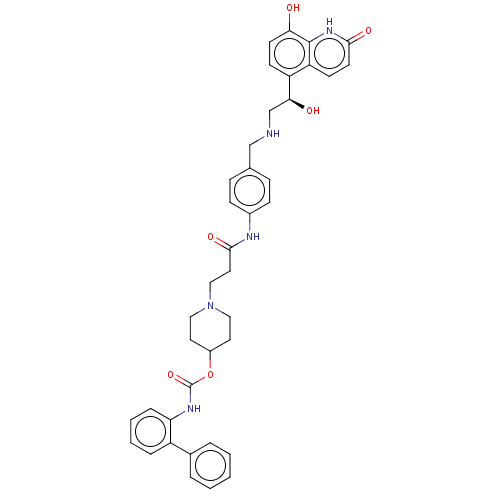 (CHEMBL3426687) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084432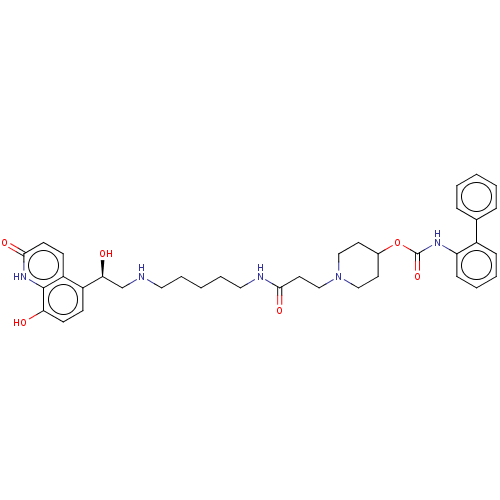 (CHEMBL3426697) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50337878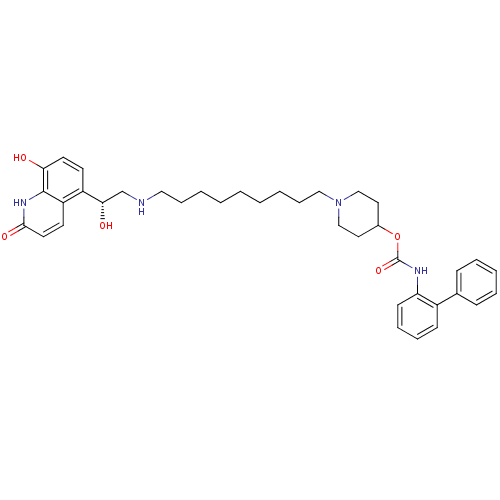 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084439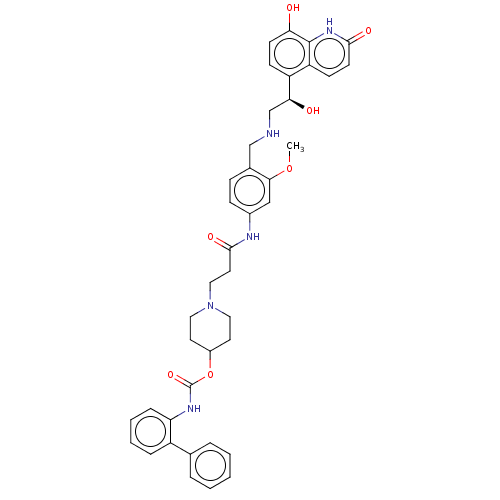 (CHEMBL3426691) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084424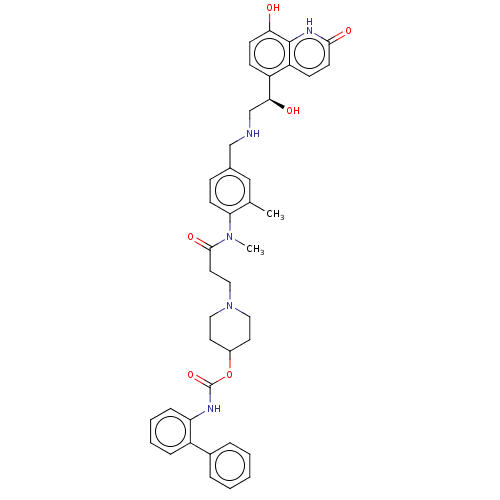 (CHEMBL3426705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084442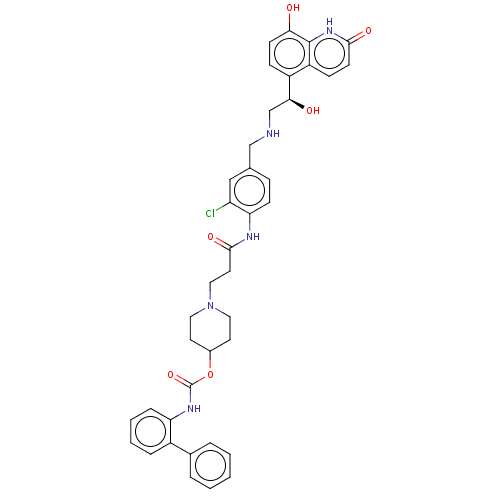 (CHEMBL3426688) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084440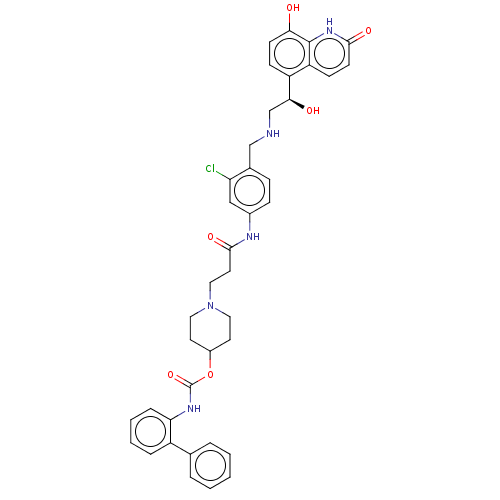 (CHEMBL3426690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084431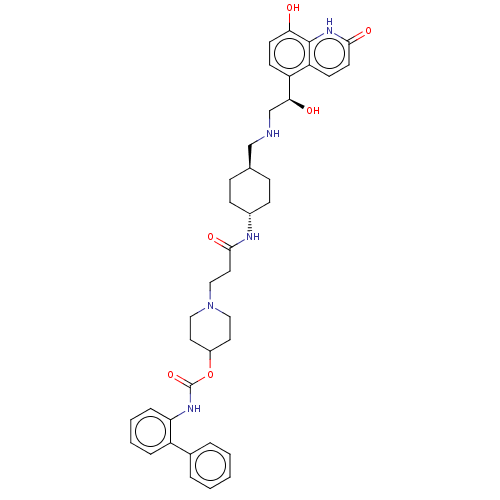 (CHEMBL3426698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084433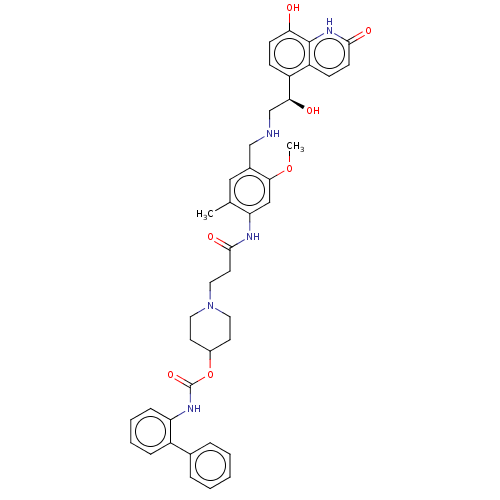 (CHEMBL3426696) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084426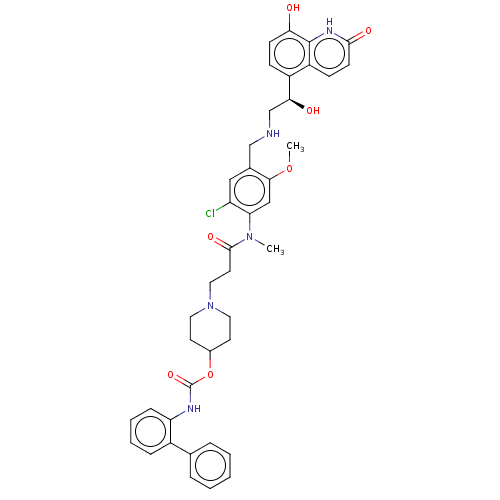 (CHEMBL3426703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084434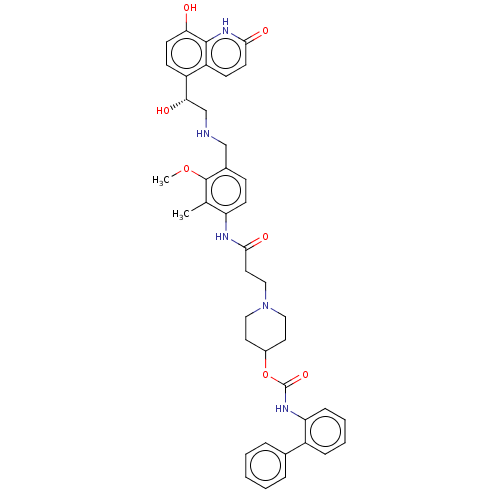 (CHEMBL3426695) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084425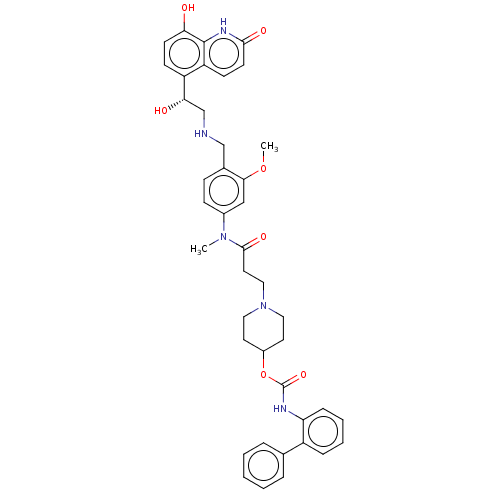 (CHEMBL3426704 | US9394275, I-25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084438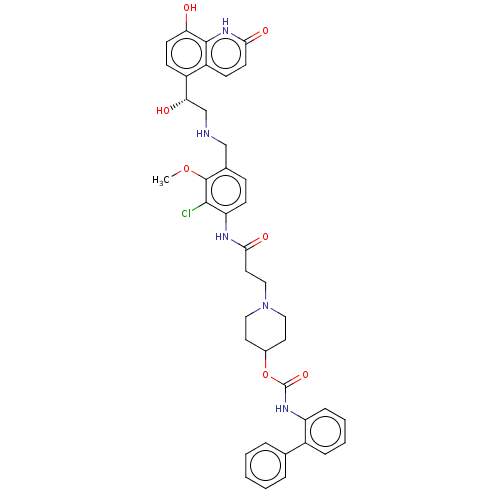 (CHEMBL3426692) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084430 (CHEMBL3426699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084427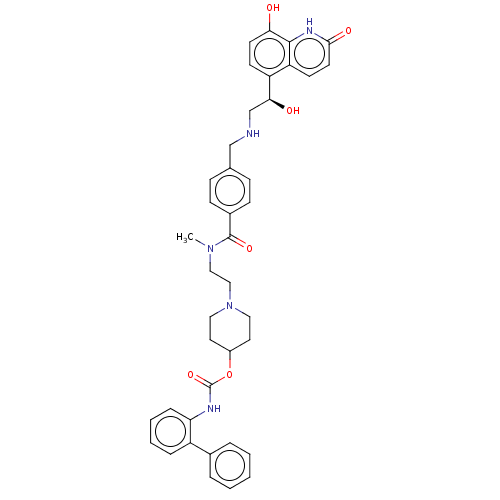 (CHEMBL3426702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084423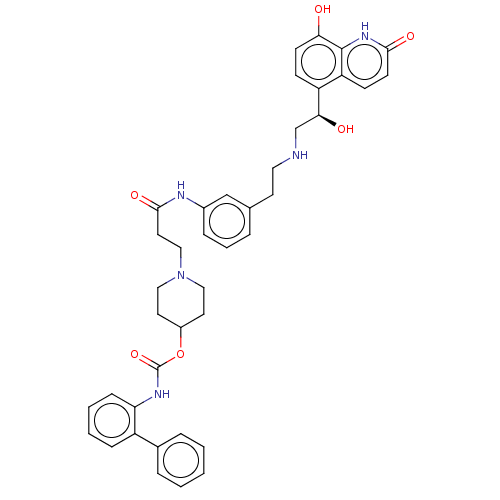 (CHEMBL3426706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084435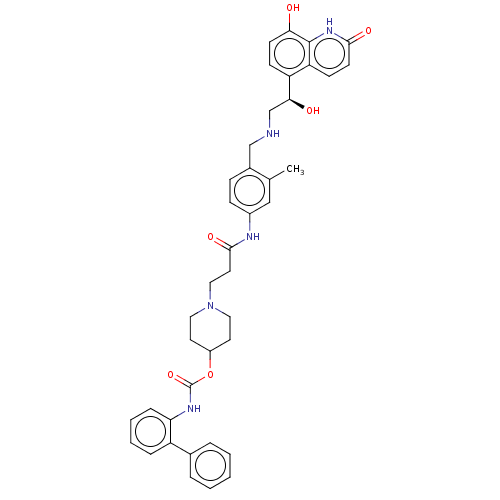 (CHEMBL3426694) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084441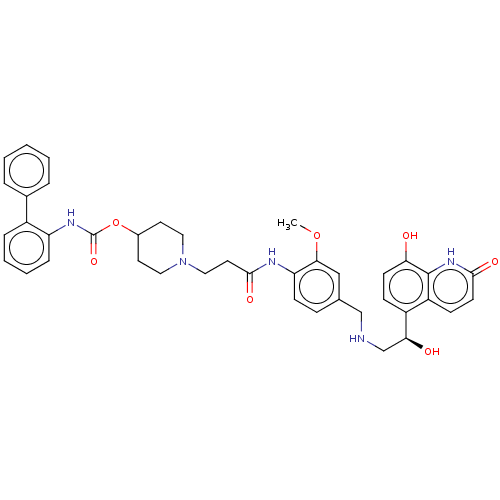 (CHEMBL3426689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084437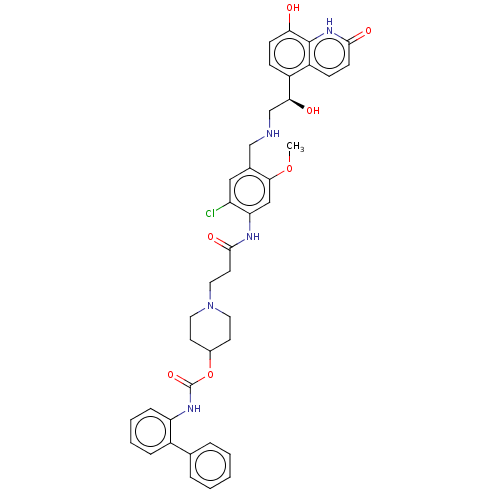 (Batefenterol | GSK961081 | GSK961081A | TD-5959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084421 (CHEMBL3426708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084428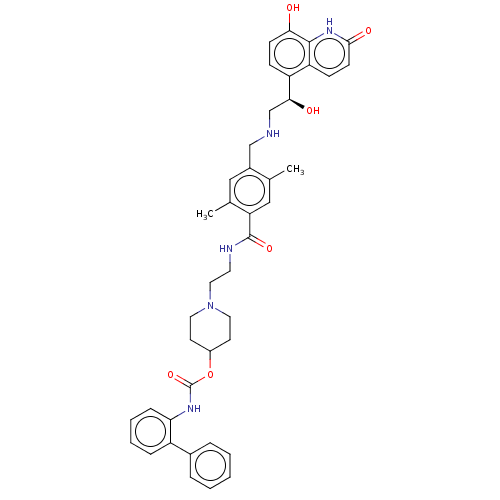 (CHEMBL3426701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084429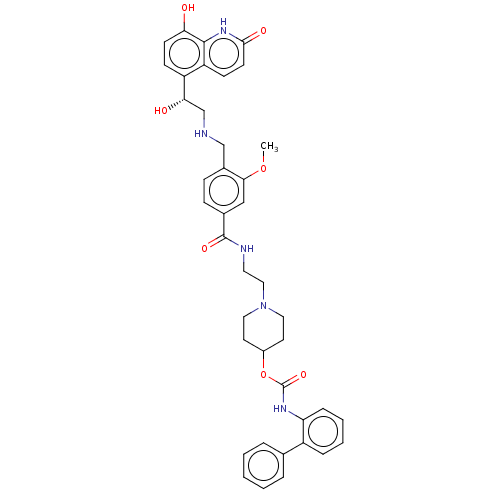 (CHEMBL3426700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50084422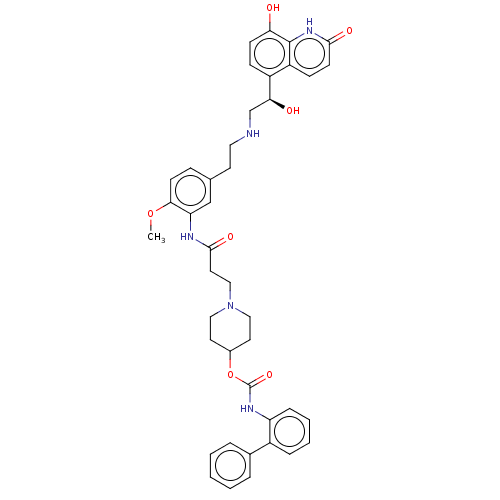 (CHEMBL3426707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50318159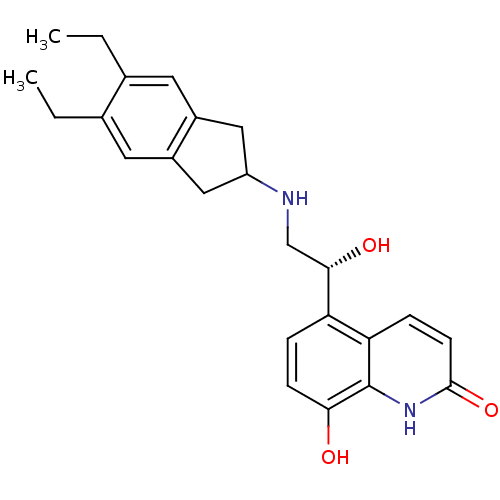 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressing CHO-K1 cells incubated for 60 mins or 6 hrs by liquid scintillation counting | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084443 (CHEMBL3426687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084442 (CHEMBL3426688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084441 (CHEMBL3426689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084440 (CHEMBL3426690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084439 (CHEMBL3426691) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084438 (CHEMBL3426692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084437 (Batefenterol | GSK961081 | GSK961081A | TD-5959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084436 (CHEMBL3426693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084435 (CHEMBL3426694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084434 (CHEMBL3426695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084433 (CHEMBL3426696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50337878 ((R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084432 (CHEMBL3426697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084431 (CHEMBL3426698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50318159 (8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084430 (CHEMBL3426699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084429 (CHEMBL3426700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084428 (CHEMBL3426701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084427 (CHEMBL3426702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084426 (CHEMBL3426703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084425 (CHEMBL3426704 | US9394275, I-25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084424 (CHEMBL3426705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50084423 (CHEMBL3426706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Theravance Biopharma, Inc. Curated by ChEMBL | Assay Description Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation countin... | J Med Chem 58: 2609-22 (2015) Article DOI: 10.1021/jm501915g BindingDB Entry DOI: 10.7270/Q2N29ZNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |