 Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50045838
Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50045838 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50421256
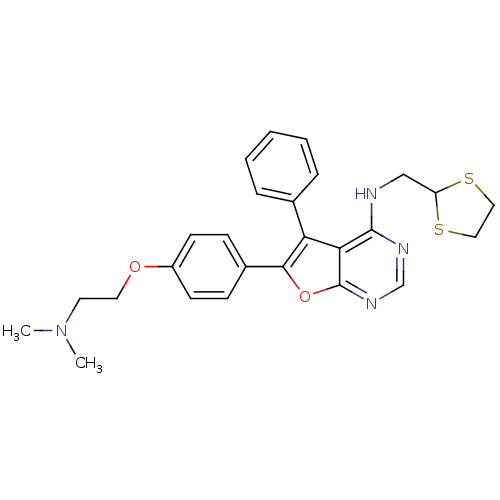
(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50421256

(CHEMBL2087874)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NCC3SCCS3)c2c1-c1ccccc1 Show InChI InChI=1S/C26H28N4O2S2/c1-30(2)12-13-31-20-10-8-19(9-11-20)24-22(18-6-4-3-5-7-18)23-25(28-17-29-26(23)32-24)27-16-21-33-14-15-34-21/h3-11,17,21H,12-16H2,1-2H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LCK using peptide poly[Glu:Tyr] (4:1) substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246164
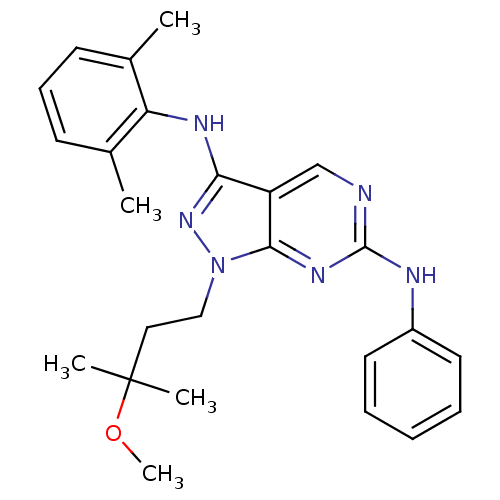
(CHEMBL487242 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccccc3)nc12 Show InChI InChI=1S/C25H30N6O/c1-17-10-9-11-18(2)21(17)28-22-20-16-26-24(27-19-12-7-6-8-13-19)29-23(20)31(30-22)15-14-25(3,4)32-5/h6-13,16H,14-15H2,1-5H3,(H,28,30)(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50084464
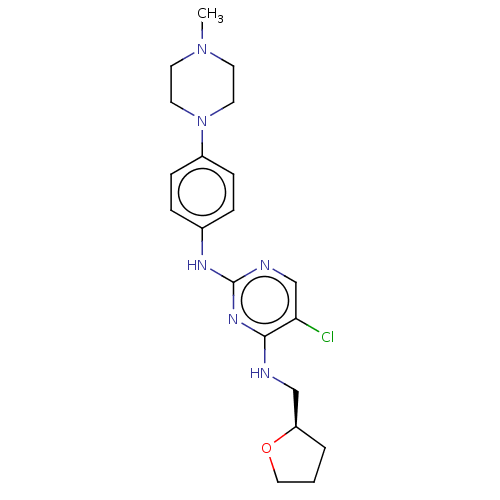
(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human TYK2 using KKSRGDYMTMQIG peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204587
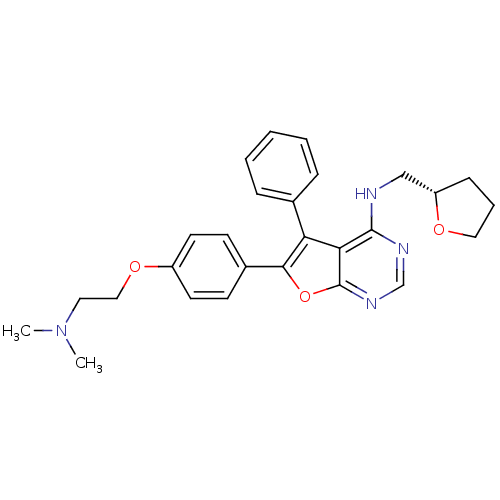
((S)-6-(4-(2-(dimethylamino)ethoxy)phenyl)-5-phenyl...)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C27H30N4O3/c1-31(2)14-16-33-21-12-10-20(11-13-21)25-23(19-7-4-3-5-8-19)24-26(29-18-30-27(24)34-25)28-17-22-9-6-15-32-22/h3-5,7-8,10-13,18,22H,6,9,14-17H2,1-2H3,(H,28,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LCK using peptide poly[Glu:Tyr] (4:1) substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 using poly[Glu:Tyr] (4:1) peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50204587

((S)-6-(4-(2-(dimethylamino)ethoxy)phenyl)-5-phenyl...)Show SMILES CN(C)CCOc1ccc(cc1)-c1oc2ncnc(NC[C@@H]3CCCO3)c2c1-c1ccccc1 Show InChI InChI=1S/C27H30N4O3/c1-31(2)14-16-33-21-12-10-20(11-13-21)25-23(19-7-4-3-5-8-19)24-26(29-18-30-27(24)34-25)28-17-22-9-6-15-32-22/h3-5,7-8,10-13,18,22H,6,9,14-17H2,1-2H3,(H,28,29,30)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50246162
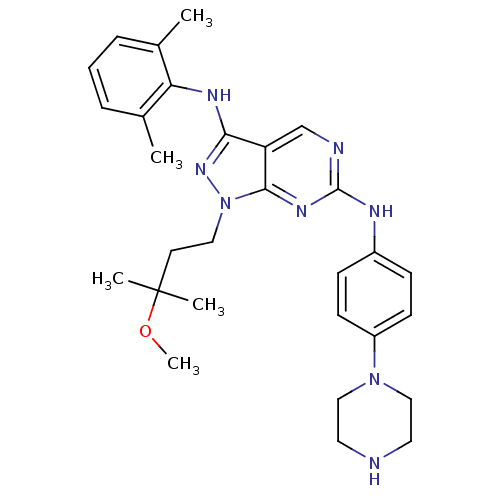
(CHEMBL472392 | N3-(2,6-dimethylphenyl)-1-(3-methox...)Show SMILES COC(C)(C)CCn1nc(Nc2c(C)cccc2C)c2cnc(Nc3ccc(cc3)N3CCNCC3)nc12 Show InChI InChI=1S/C29H38N8O/c1-20-7-6-8-21(2)25(20)33-26-24-19-31-28(34-27(24)37(35-26)16-13-29(3,4)38-5)32-22-9-11-23(12-10-22)36-17-14-30-15-18-36/h6-12,19,30H,13-18H2,1-5H3,(H,33,35)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084463
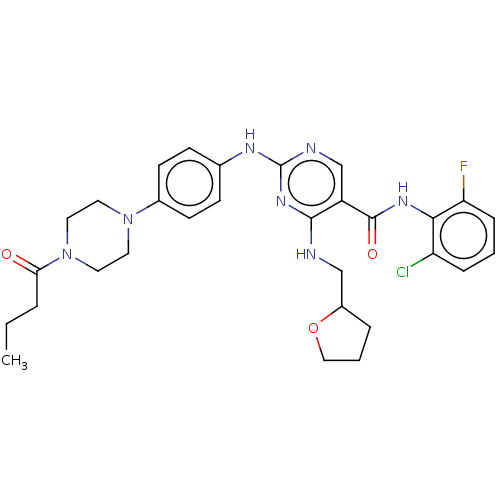
(CHEMBL3426880)Show SMILES CCCC(=O)N1CCN(CC1)c1ccc(Nc2ncc(C(=O)Nc3c(F)cccc3Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C30H35ClFN7O3/c1-2-5-26(40)39-15-13-38(14-16-39)21-11-9-20(10-12-21)35-30-34-19-23(28(37-30)33-18-22-6-4-17-42-22)29(41)36-27-24(31)7-3-8-25(27)32/h3,7-12,19,22H,2,4-6,13-18H2,1H3,(H,36,41)(H2,33,34,35,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084454
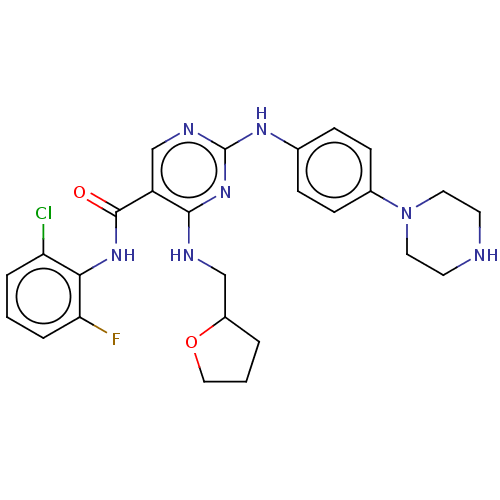
(CHEMBL3426870)Show SMILES Fc1cccc(Cl)c1NC(=O)c1cnc(Nc2ccc(cc2)N2CCNCC2)nc1NCC1CCCO1 Show InChI InChI=1S/C26H29ClFN7O2/c27-21-4-1-5-22(28)23(21)33-25(36)20-16-31-26(34-24(20)30-15-19-3-2-14-37-19)32-17-6-8-18(9-7-17)35-12-10-29-11-13-35/h1,4-9,16,19,29H,2-3,10-15H2,(H,33,36)(H2,30,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084462
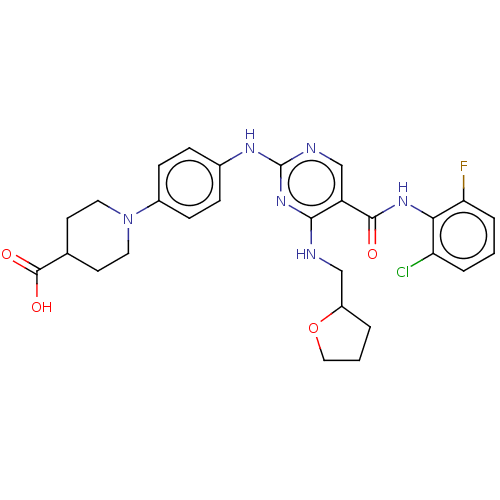
(CHEMBL3426879)Show SMILES OC(=O)C1CCN(CC1)c1ccc(Nc2ncc(C(=O)Nc3c(F)cccc3Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C28H30ClFN6O4/c29-22-4-1-5-23(30)24(22)34-26(37)21-16-32-28(35-25(21)31-15-20-3-2-14-40-20)33-18-6-8-19(9-7-18)36-12-10-17(11-13-36)27(38)39/h1,4-9,16-17,20H,2-3,10-15H2,(H,34,37)(H,38,39)(H2,31,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM98282
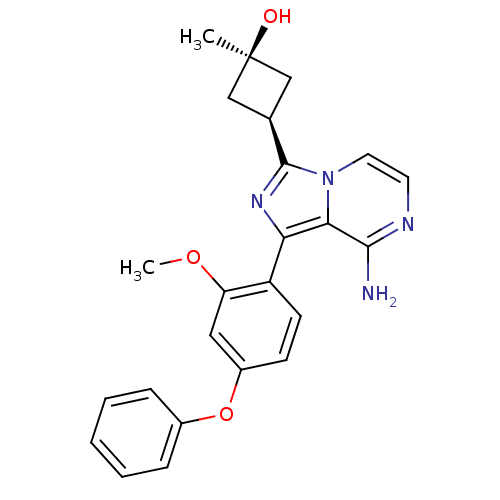
(US8481733, 94)Show SMILES COc1cc(Oc2ccccc2)ccc1-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12 |r,wU:18.19,20.23,wD:20.22,(2.01,-1.15,;2.01,.39,;.92,1.48,;1.32,2.96,;.23,4.05,;.63,5.54,;2.12,5.94,;3.21,4.85,;4.7,5.25,;5.09,6.74,;4.01,7.83,;2.52,7.43,;-1.25,3.65,;-1.65,2.17,;-.56,1.08,;-.96,-.41,;-.06,-1.65,;-.96,-2.9,;-.56,-4.39,;.77,-5.16,;-0,-6.49,;1.33,-7.26,;-.77,-7.83,;-1.33,-5.72,;-2.43,-2.42,;-3.76,-3.19,;-5.09,-2.42,;-5.09,-.88,;-3.76,-.11,;-3.76,1.43,;-2.43,-.88,)| Show InChI InChI=1S/C24H24N4O3/c1-24(29)13-15(14-24)23-27-20(21-22(25)26-10-11-28(21)23)18-9-8-17(12-19(18)30-2)31-16-6-4-3-5-7-16/h3-12,15,29H,13-14H2,1-2H3,(H2,25,26)/t15-,24+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) by cell-based assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084457
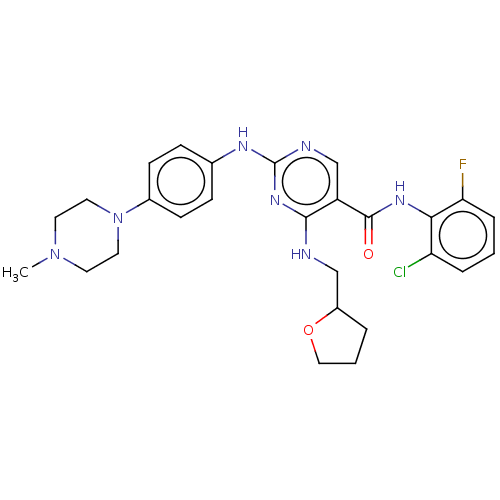
(CHEMBL3426873)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(C(=O)Nc3c(F)cccc3Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C27H31ClFN7O2/c1-35-11-13-36(14-12-35)19-9-7-18(8-10-19)32-27-31-17-21(25(34-27)30-16-20-4-3-15-38-20)26(37)33-24-22(28)5-2-6-23(24)29/h2,5-10,17,20H,3-4,11-16H2,1H3,(H,33,37)(H2,30,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084456

(CHEMBL3426872)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Br)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C20H27BrN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084451
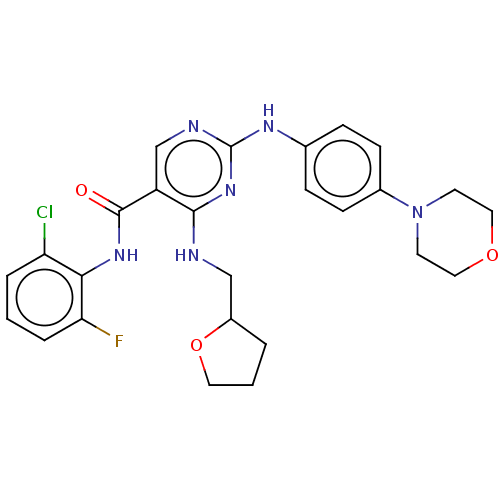
(CHEMBL3426867)Show SMILES Fc1cccc(Cl)c1NC(=O)c1cnc(Nc2ccc(cc2)N2CCOCC2)nc1NCC1CCCO1 Show InChI InChI=1S/C26H28ClFN6O3/c27-21-4-1-5-22(28)23(21)32-25(35)20-16-30-26(33-24(20)29-15-19-3-2-12-37-19)31-17-6-8-18(9-7-17)34-10-13-36-14-11-34/h1,4-9,16,19H,2-3,10-15H2,(H,32,35)(H2,29,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084460
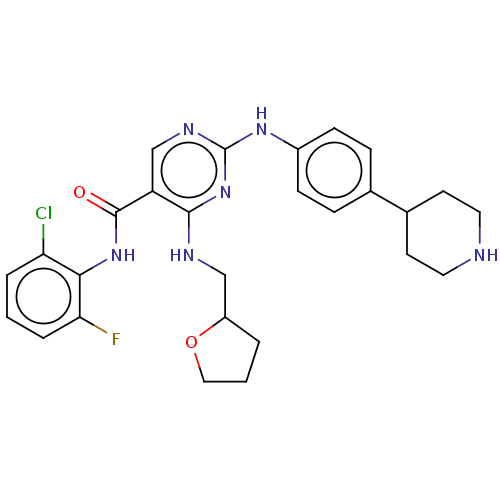
(CHEMBL3426876)Show SMILES Fc1cccc(Cl)c1NC(=O)c1cnc(Nc2ccc(cc2)C2CCNCC2)nc1NCC1CCCO1 Show InChI InChI=1S/C27H30ClFN6O2/c28-22-4-1-5-23(29)24(22)34-26(36)21-16-32-27(35-25(21)31-15-20-3-2-14-37-20)33-19-8-6-17(7-9-19)18-10-12-30-13-11-18/h1,4-9,16,18,20,30H,2-3,10-15H2,(H,34,36)(H2,31,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084447
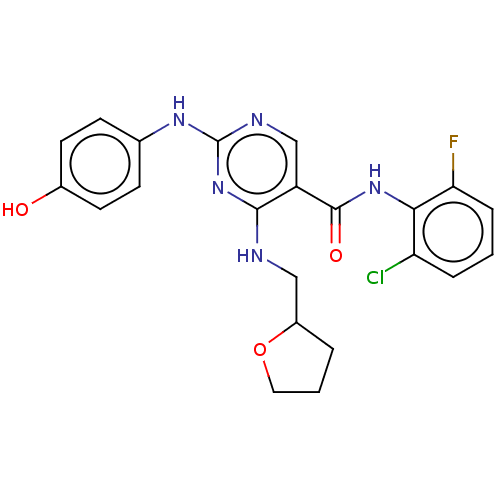
(CHEMBL3426861)Show SMILES Oc1ccc(Nc2ncc(C(=O)Nc3c(F)cccc3Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C22H21ClFN5O3/c23-17-4-1-5-18(24)19(17)28-21(31)16-12-26-22(27-13-6-8-14(30)9-7-13)29-20(16)25-11-15-3-2-10-32-15/h1,4-9,12,15,30H,2-3,10-11H2,(H,28,31)(H2,25,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084455
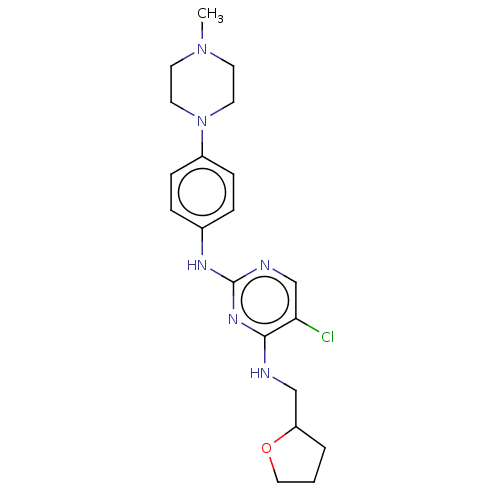
(CHEMBL3426871)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084449
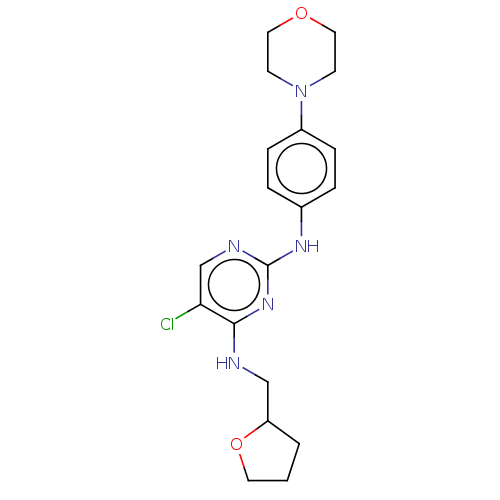
(CHEMBL3426865)Show InChI InChI=1S/C19H24ClN5O2/c20-17-13-22-19(24-18(17)21-12-16-2-1-9-27-16)23-14-3-5-15(6-4-14)25-7-10-26-11-8-25/h3-6,13,16H,1-2,7-12H2,(H2,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084452
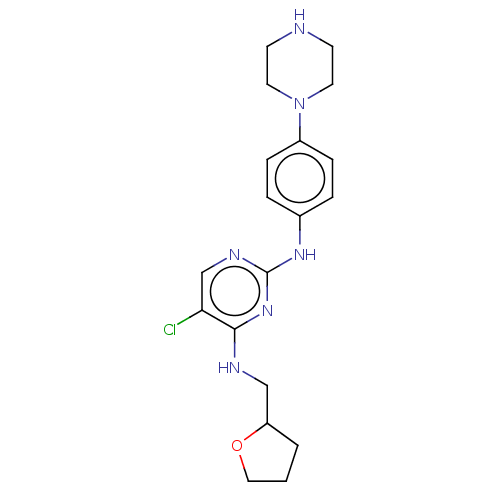
(CHEMBL3426868)Show InChI InChI=1S/C19H25ClN6O/c20-17-13-23-19(25-18(17)22-12-16-2-1-11-27-16)24-14-3-5-15(6-4-14)26-9-7-21-8-10-26/h3-6,13,16,21H,1-2,7-12H2,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084465
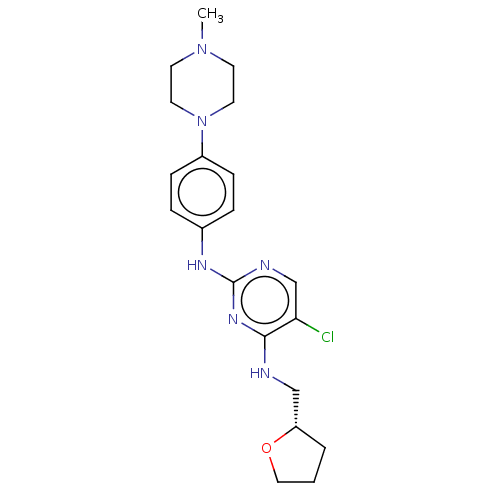
(CHEMBL3426892)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084461
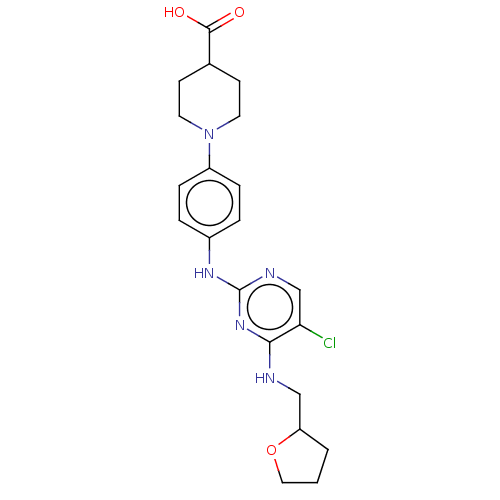
(CHEMBL3426877)Show SMILES OC(=O)C1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NCC3CCCO3)n2)cc1 Show InChI InChI=1S/C21H26ClN5O3/c22-18-13-24-21(26-19(18)23-12-17-2-1-11-30-17)25-15-3-5-16(6-4-15)27-9-7-14(8-10-27)20(28)29/h3-6,13-14,17H,1-2,7-12H2,(H,28,29)(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084453
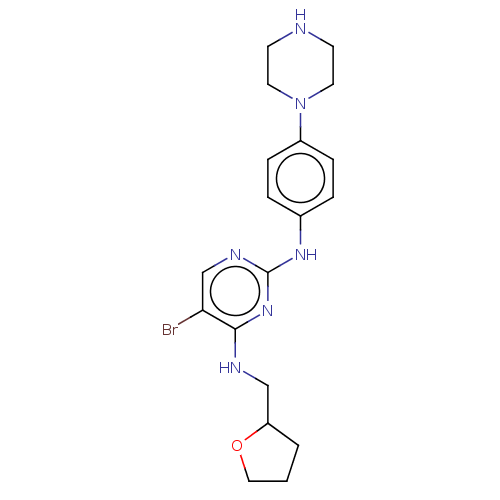
(CHEMBL3426869)Show InChI InChI=1S/C19H25BrN6O/c20-17-13-23-19(25-18(17)22-12-16-2-1-11-27-16)24-14-3-5-15(6-4-14)26-9-7-21-8-10-26/h3-6,13,16,21H,1-2,7-12H2,(H2,22,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084448
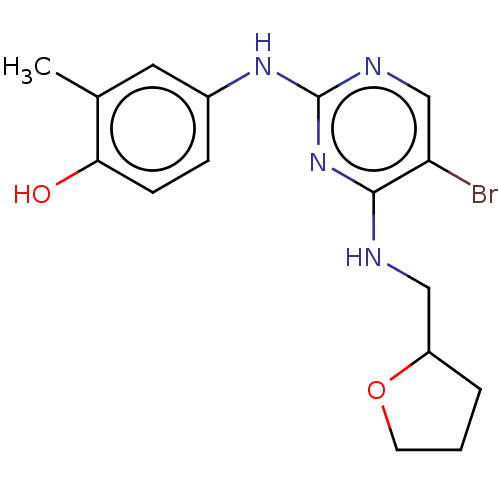
(CHEMBL3426862)Show InChI InChI=1S/C16H19BrN4O2/c1-10-7-11(4-5-14(10)22)20-16-19-9-13(17)15(21-16)18-8-12-3-2-6-23-12/h4-5,7,9,12,22H,2-3,6,8H2,1H3,(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084450
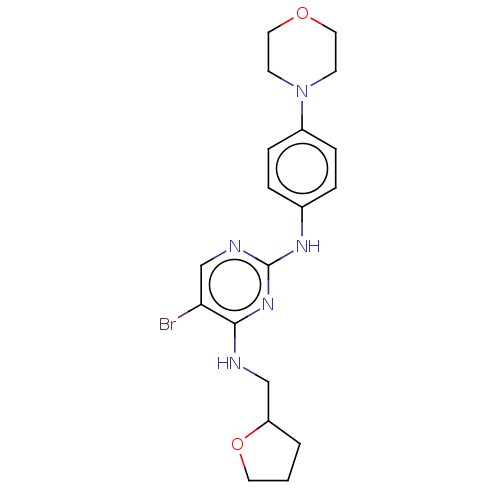
(CHEMBL3426866)Show InChI InChI=1S/C19H24BrN5O2/c20-17-13-22-19(24-18(17)21-12-16-2-1-9-27-16)23-14-3-5-15(6-4-14)25-7-10-26-11-8-25/h3-6,13,16H,1-2,7-12H2,(H2,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM98282

(US8481733, 94)Show SMILES COc1cc(Oc2ccccc2)ccc1-c1nc([C@H]2C[C@@](C)(O)C2)n2ccnc(N)c12 |r,wU:18.19,20.23,wD:20.22,(2.01,-1.15,;2.01,.39,;.92,1.48,;1.32,2.96,;.23,4.05,;.63,5.54,;2.12,5.94,;3.21,4.85,;4.7,5.25,;5.09,6.74,;4.01,7.83,;2.52,7.43,;-1.25,3.65,;-1.65,2.17,;-.56,1.08,;-.96,-.41,;-.06,-1.65,;-.96,-2.9,;-.56,-4.39,;.77,-5.16,;-0,-6.49,;1.33,-7.26,;-.77,-7.83,;-1.33,-5.72,;-2.43,-2.42,;-3.76,-3.19,;-5.09,-2.42,;-5.09,-.88,;-3.76,-.11,;-3.76,1.43,;-2.43,-.88,)| Show InChI InChI=1S/C24H24N4O3/c1-24(29)13-15(14-24)23-27-20(21-22(25)26-10-11-28(21)23)18-9-8-17(12-19(18)30-2)31-16-6-4-3-5-7-16/h3-12,15,29H,13-14H2,1-2H3,(H2,25,26)/t15-,24+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50204589
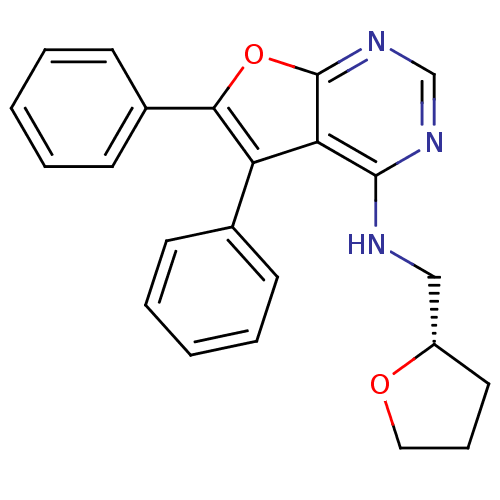
((S)-5,6-diphenyl-N-((tetrahydrofuran-2-yl)methyl)f...)Show SMILES C(Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1)[C@@H]1CCCO1 Show InChI InChI=1S/C23H21N3O2/c1-3-8-16(9-4-1)19-20-22(24-14-18-12-7-13-27-18)25-15-26-23(20)28-21(19)17-10-5-2-6-11-17/h1-6,8-11,15,18H,7,12-14H2,(H,24,25,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LCK using peptide poly[Glu:Tyr] (4:1) substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ROS/ROS1 using KKKSPGEYVNIEFG peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human LCK using peptide poly[Glu:Tyr] (4:1) substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ALK using poly[Glu:Tyr] (4:1) peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human CHK1 using KKKVSRSGLYRSPSMPENLNRPR peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human FGFR1 using KKKSPGEYVNIEFG peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084446
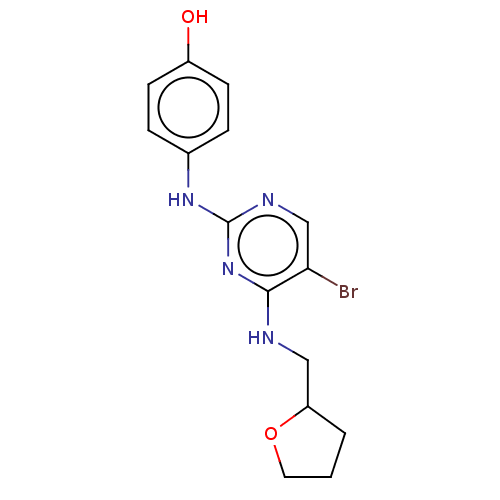
(CHEMBL3426860)Show InChI InChI=1S/C15H17BrN4O2/c16-13-9-18-15(19-10-3-5-11(21)6-4-10)20-14(13)17-8-12-2-1-7-22-12/h3-6,9,12,21H,1-2,7-8H2,(H2,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
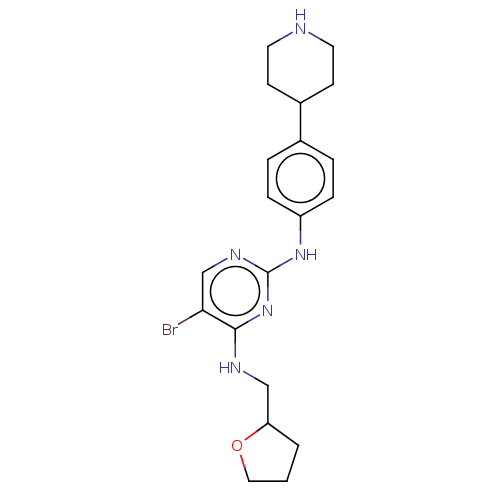
(Homo sapiens (Human)) | BDBM50084459
(CHEMBL3426875)Show InChI InChI=1S/C20H26BrN5O/c21-18-13-24-20(26-19(18)23-12-17-2-1-11-27-17)25-16-5-3-14(4-6-16)15-7-9-22-10-8-15/h3-6,13,15,17,22H,1-2,7-12H2,(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ABL1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084458
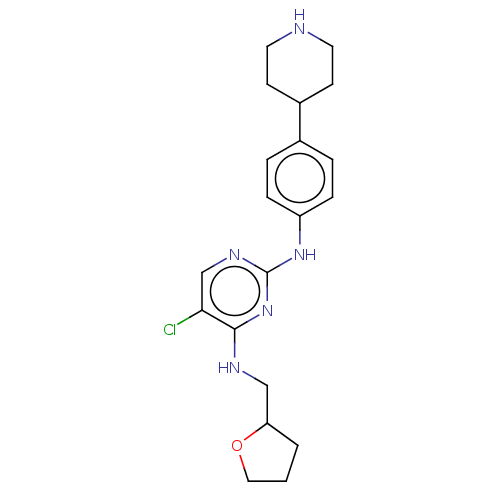
(CHEMBL3426874)Show InChI InChI=1S/C20H26ClN5O/c21-18-13-24-20(26-19(18)23-12-17-2-1-11-27-17)25-16-5-3-14(4-6-16)15-7-9-22-10-8-15/h3-6,13,15,17,22H,1-2,7-12H2,(H2,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50084444
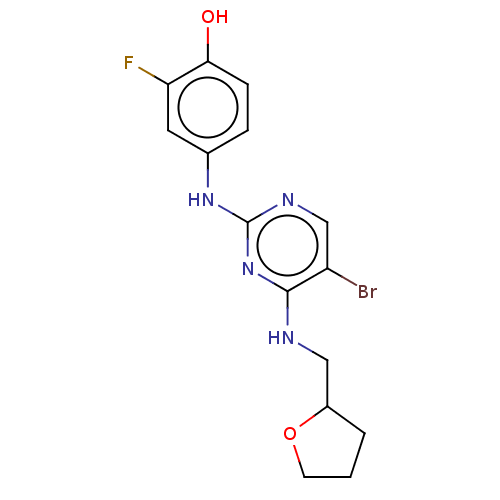
(CHEMBL3426857)Show InChI InChI=1S/C15H16BrFN4O2/c16-11-8-19-15(20-9-3-4-13(22)12(17)6-9)21-14(11)18-7-10-2-1-5-23-10/h3-4,6,8,10,22H,1-2,5,7H2,(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 299 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human ACK1 using EAIYAAPFAKKK peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50084464

(CHEMBL3426891)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(Cl)c(NC[C@H]3CCCO3)n2)cc1 |r| Show InChI InChI=1S/C20H27ClN6O/c1-26-8-10-27(11-9-26)16-6-4-15(5-7-16)24-20-23-14-18(21)19(25-20)22-13-17-3-2-12-28-17/h4-7,14,17H,2-3,8-13H2,1H3,(H2,22,23,24,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of human c-Src using poly[Glu:Tyr] (4:1) peptide substrate by 33P Hotspot assay |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50337126
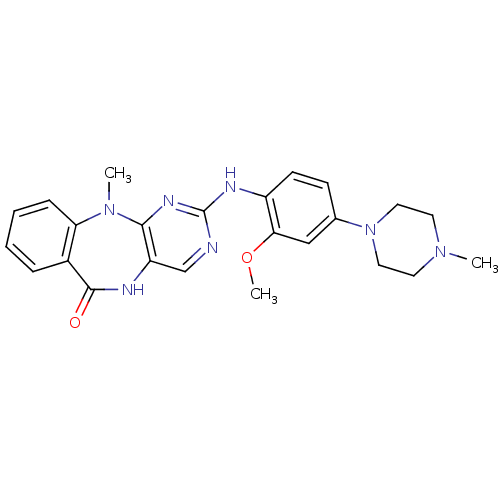
(2-(2-methoxy-4-(4-methylpiperazin-1-yl)phenylamino...)Show SMILES COc1cc(ccc1Nc1ncc2NC(=O)c3ccccc3N(C)c2n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O2/c1-29-10-12-31(13-11-29)16-8-9-18(21(14-16)33-3)27-24-25-15-19-22(28-24)30(2)20-7-5-4-6-17(20)23(32)26-19/h4-9,14-15H,10-13H2,1-3H3,(H,26,32)(H,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
University of South Florida
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 kinase (unknown origin) |
J Med Chem 58: 2746-63 (2015)
Article DOI: 10.1021/jm501929n
BindingDB Entry DOI: 10.7270/Q2H996XC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data