 Found 23 hits Enz. Inhib. hit(s) with all data for entry = 50017459
Found 23 hits Enz. Inhib. hit(s) with all data for entry = 50017459 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP1 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Stromelysin-2
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP10 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP7 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of MMP14 |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50182409
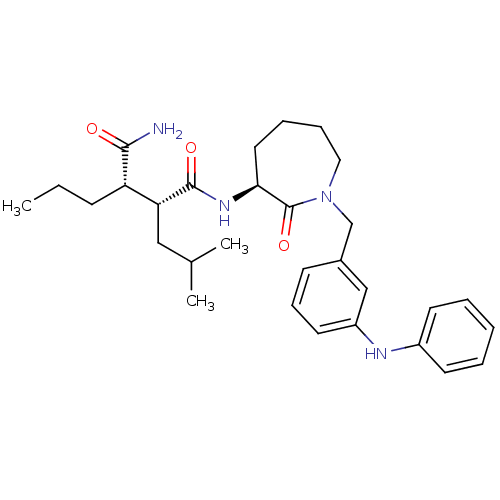
((2R,3S)-N1-((S)-1-(3-(phenylamino)benzyl)-2-oxoaze...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccccc3)c2)C1=O)C(N)=O Show InChI InChI=1S/C30H42N4O3/c1-4-11-25(28(31)35)26(18-21(2)3)29(36)33-27-16-8-9-17-34(30(27)37)20-22-12-10-15-24(19-22)32-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,32H,4,8-9,11,16-18,20H2,1-3H3,(H2,31,35)(H,33,36)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410871
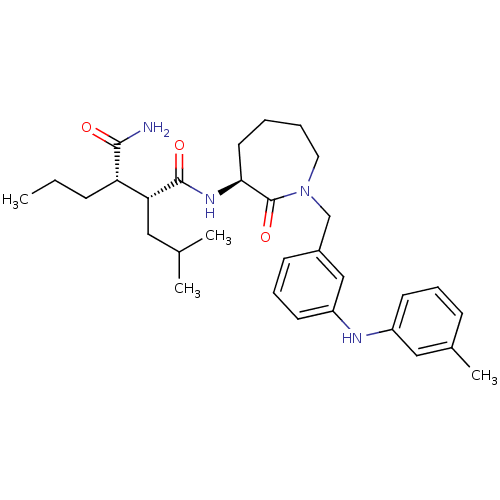
(CHEMBL208350)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3cccc(C)c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H44N4O3/c1-5-10-26(29(32)36)27(17-21(2)3)30(37)34-28-15-6-7-16-35(31(28)38)20-23-12-9-14-25(19-23)33-24-13-8-11-22(4)18-24/h8-9,11-14,18-19,21,26-28,33H,5-7,10,15-17,20H2,1-4H3,(H2,32,36)(H,34,37)/t26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410864
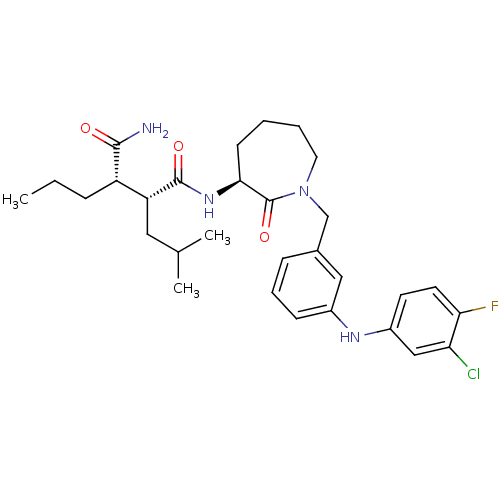
(CHEMBL208504)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccc(F)c(Cl)c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C30H40ClFN4O3/c1-4-8-23(28(33)37)24(15-19(2)3)29(38)35-27-11-5-6-14-36(30(27)39)18-20-9-7-10-21(16-20)34-22-12-13-26(32)25(31)17-22/h7,9-10,12-13,16-17,19,23-24,27,34H,4-6,8,11,14-15,18H2,1-3H3,(H2,33,37)(H,35,38)/t23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410859
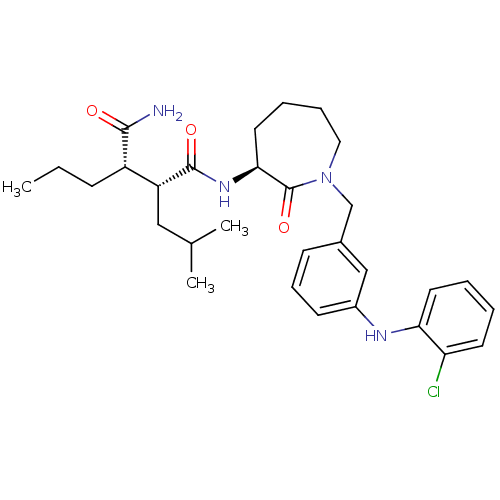
(CHEMBL382880)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccccc3Cl)c2)C1=O)C(N)=O Show InChI InChI=1S/C30H41ClN4O3/c1-4-10-23(28(32)36)24(17-20(2)3)29(37)34-27-15-7-8-16-35(30(27)38)19-21-11-9-12-22(18-21)33-26-14-6-5-13-25(26)31/h5-6,9,11-14,18,20,23-24,27,33H,4,7-8,10,15-17,19H2,1-3H3,(H2,32,36)(H,34,37)/t23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410861
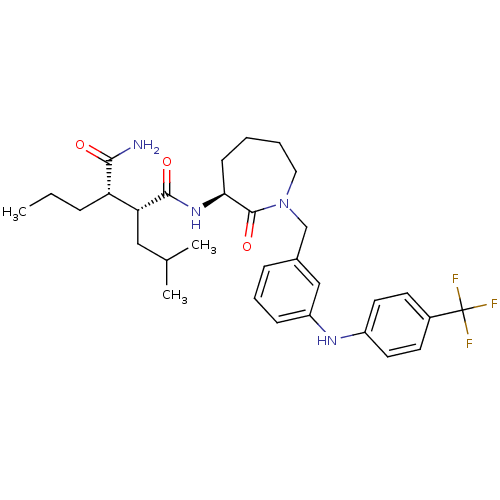
(CHEMBL424684)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccc(cc3)C(F)(F)F)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H41F3N4O3/c1-4-8-25(28(35)39)26(17-20(2)3)29(40)37-27-11-5-6-16-38(30(27)41)19-21-9-7-10-24(18-21)36-23-14-12-22(13-15-23)31(32,33)34/h7,9-10,12-15,18,20,25-27,36H,4-6,8,11,16-17,19H2,1-3H3,(H2,35,39)(H,37,40)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410860
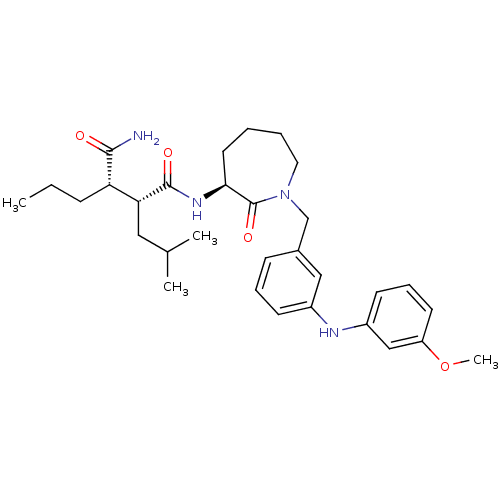
(CHEMBL410532)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3cccc(OC)c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H44N4O4/c1-5-10-26(29(32)36)27(17-21(2)3)30(37)34-28-15-6-7-16-35(31(28)38)20-22-11-8-12-23(18-22)33-24-13-9-14-25(19-24)39-4/h8-9,11-14,18-19,21,26-28,33H,5-7,10,15-17,20H2,1-4H3,(H2,32,36)(H,34,37)/t26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410863
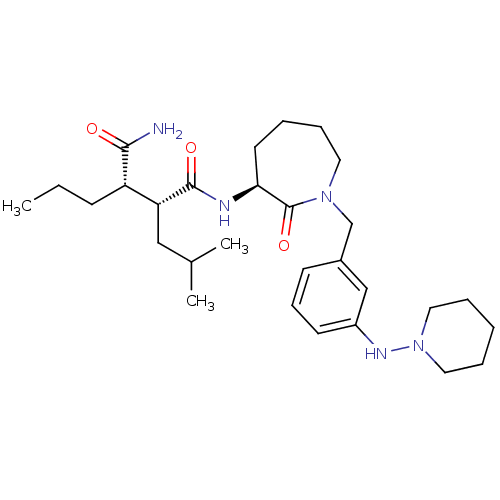
(CHEMBL208230)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(NN3CCCCC3)c2)C1=O)C(N)=O Show InChI InChI=1S/C29H47N5O3/c1-4-11-24(27(30)35)25(18-21(2)3)28(36)31-26-14-6-9-15-33(29(26)37)20-22-12-10-13-23(19-22)32-34-16-7-5-8-17-34/h10,12-13,19,21,24-26,32H,4-9,11,14-18,20H2,1-3H3,(H2,30,35)(H,31,36)/t24-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410868
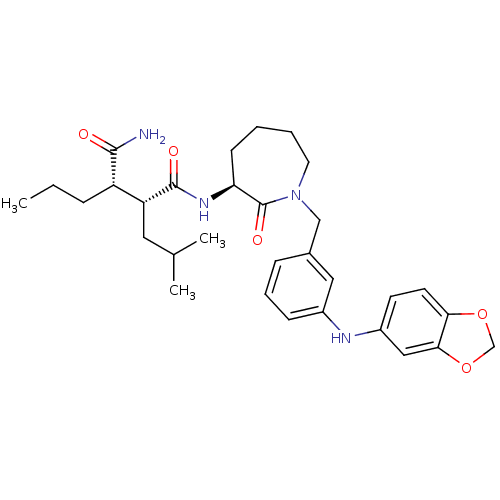
(CHEMBL205970)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccc4OCOc4c3)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H42N4O5/c1-4-8-24(29(32)36)25(15-20(2)3)30(37)34-26-11-5-6-14-35(31(26)38)18-21-9-7-10-22(16-21)33-23-12-13-27-28(17-23)40-19-39-27/h7,9-10,12-13,16-17,20,24-26,33H,4-6,8,11,14-15,18-19H2,1-3H3,(H2,32,36)(H,34,37)/t24-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410867
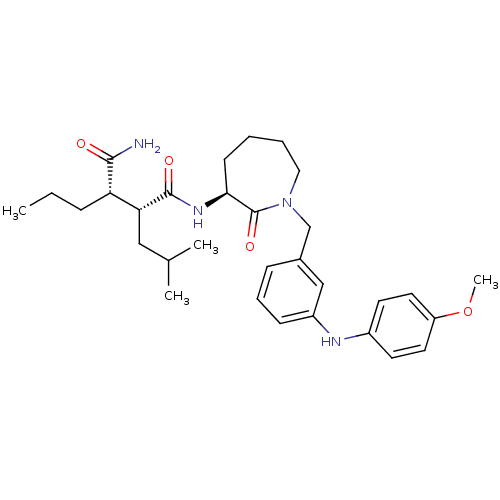
(CHEMBL274414)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Nc3ccc(OC)cc3)c2)C1=O)C(N)=O Show InChI InChI=1S/C31H44N4O4/c1-5-9-26(29(32)36)27(18-21(2)3)30(37)34-28-12-6-7-17-35(31(28)38)20-22-10-8-11-24(19-22)33-23-13-15-25(39-4)16-14-23/h8,10-11,13-16,19,21,26-28,33H,5-7,9,12,17-18,20H2,1-4H3,(H2,32,36)(H,34,37)/t26-,27+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50182403

((2R,3S)-N1-((S)-1-(3-phenoxybenzyl)-2-oxoazepan-3-...)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NO Show InChI InChI=1S/C30H41N3O5/c1-4-11-25(29(35)32-37)26(18-21(2)3)28(34)31-27-16-8-9-17-33(30(27)36)20-22-12-10-15-24(19-22)38-23-13-6-5-7-14-23/h5-7,10,12-15,19,21,25-27,37H,4,8-9,11,16-18,20H2,1-3H3,(H,31,34)(H,32,35)/t25-,26+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410865
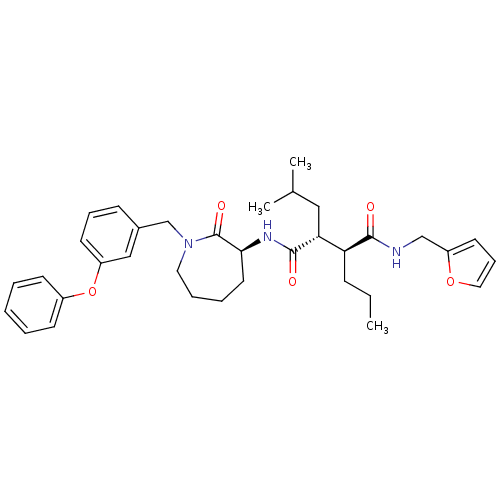
(CHEMBL205400)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NCc1ccco1 Show InChI InChI=1S/C35H45N3O5/c1-4-12-30(33(39)36-23-29-17-11-20-42-29)31(21-25(2)3)34(40)37-32-18-8-9-19-38(35(32)41)24-26-13-10-16-28(22-26)43-27-14-6-5-7-15-27/h5-7,10-11,13-17,20,22,25,30-32H,4,8-9,12,18-19,21,23-24H2,1-3H3,(H,36,39)(H,37,40)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410870
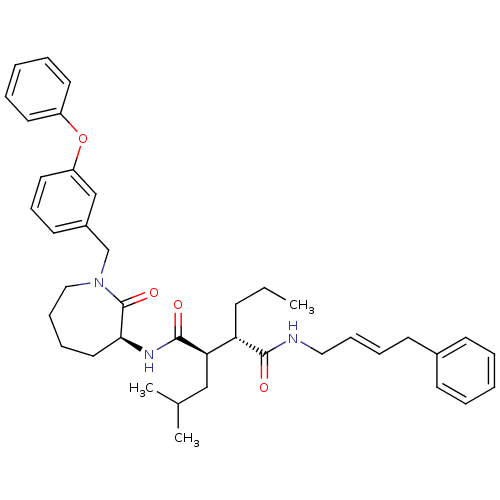
(CHEMBL204443)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NC\C=C\Cc1ccccc1 Show InChI InChI=1S/C40H51N3O4/c1-4-16-35(38(44)41-25-13-11-19-31-17-7-5-8-18-31)36(27-30(2)3)39(45)42-37-24-12-14-26-43(40(37)46)29-32-20-15-23-34(28-32)47-33-21-9-6-10-22-33/h5-11,13,15,17-18,20-23,28,30,35-37H,4,12,14,16,19,24-27,29H2,1-3H3,(H,41,44)(H,42,45)/b13-11+/t35-,36+,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410866

(CHEMBL205714)Show SMILES CCCCNC(=O)[C@@H](CCC)[C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O Show InChI InChI=1S/C34H49N3O4/c1-5-7-20-35-32(38)29(14-6-2)30(22-25(3)4)33(39)36-31-19-11-12-21-37(34(31)40)24-26-15-13-18-28(23-26)41-27-16-9-8-10-17-27/h8-10,13,15-18,23,25,29-31H,5-7,11-12,14,19-22,24H2,1-4H3,(H,35,38)(H,36,39)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410862
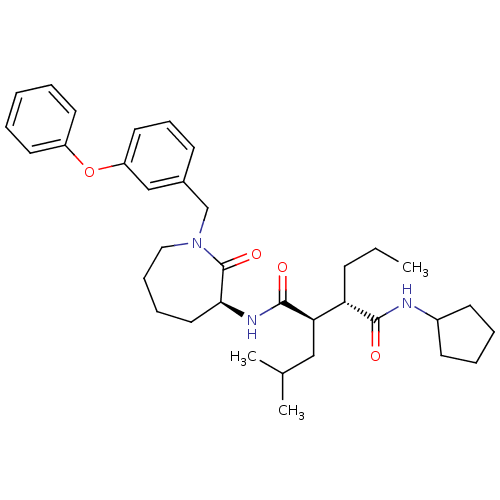
(CHEMBL381336)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2cccc(Oc3ccccc3)c2)C1=O)C(=O)NC1CCCC1 Show InChI InChI=1S/C35H49N3O4/c1-4-13-30(33(39)36-27-15-8-9-16-27)31(22-25(2)3)34(40)37-32-20-10-11-21-38(35(32)41)24-26-14-12-19-29(23-26)42-28-17-6-5-7-18-28/h5-7,12,14,17-19,23,25,27,30-32H,4,8-11,13,15-16,20-22,24H2,1-3H3,(H,36,39)(H,37,40)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50410869
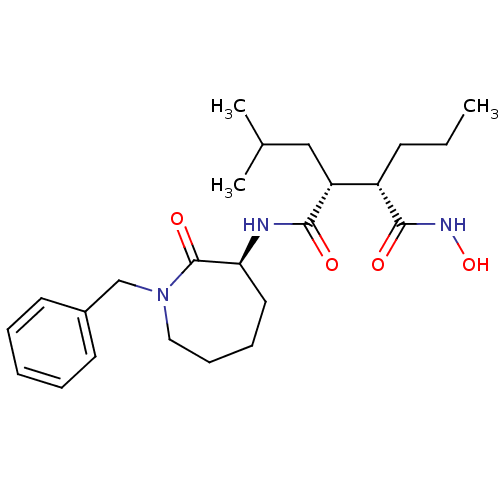
(CHEMBL206766)Show SMILES CCC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H]1CCCCN(Cc2ccccc2)C1=O)C(=O)NO Show InChI InChI=1S/C24H37N3O4/c1-4-10-19(23(29)26-31)20(15-17(2)3)22(28)25-21-13-8-9-14-27(24(21)30)16-18-11-6-5-7-12-18/h5-7,11-12,17,19-21,31H,4,8-10,13-16H2,1-3H3,(H,25,28)(H,26,29)/t19-,20+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of beta-amyloid peptide production in CHO N9 cell line |
Bioorg Med Chem Lett 16: 2357-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.055
BindingDB Entry DOI: 10.7270/Q2WD405K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data