

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186937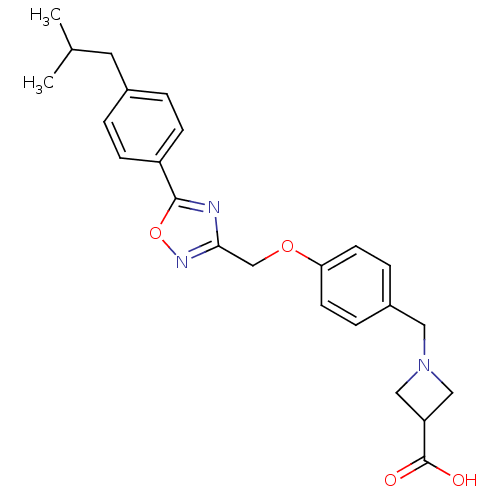 (1-(4-((5-(4-isobutylphenyl)-1,2,4-oxadiazol-3-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]S1P binding to S1P1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50158335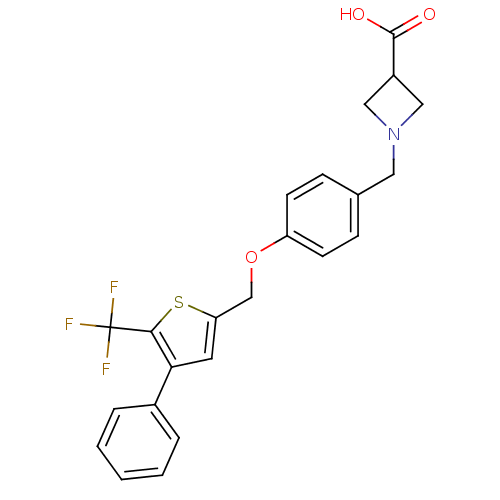 (1-(4-((4-phenyl-5-(trifluoromethyl)thiophen-2-yl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [33P]S1P binding to S1P1 receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22223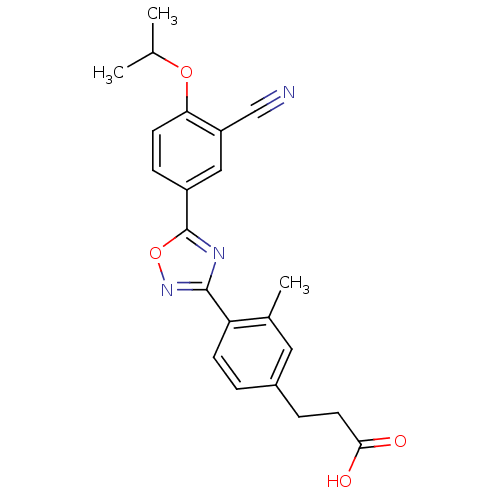 (3-(4-{5-[3-cyano-4-(propan-2-yloxy)phenyl]-1,2,4-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <0.0800 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186920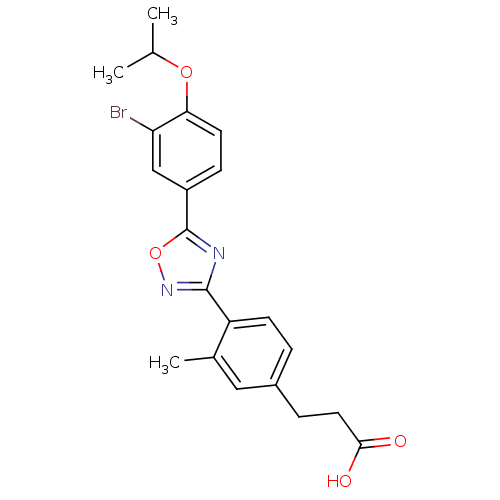 (3-(4-(5-(3-bromo-4-isopropoxyphenyl)-1,2,4-oxadiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <0.0800 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186933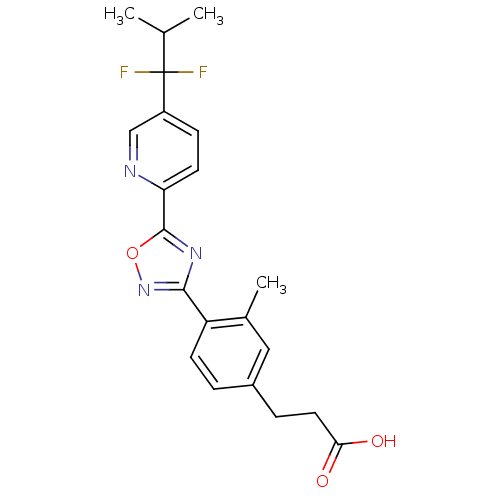 (3-(4-(5-(5-(1,1-difluoro-2-methylpropyl)pyridin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186915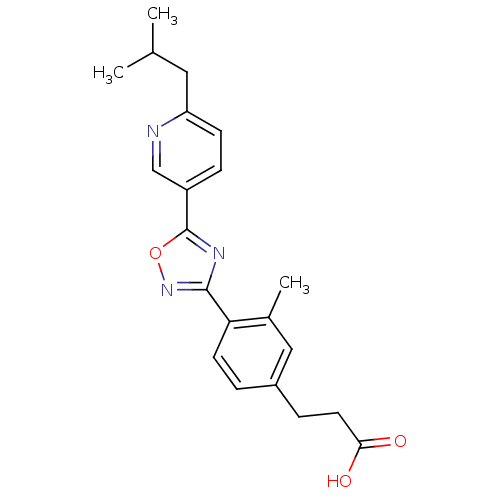 (3-(4-(5-(6-isobutylpyridin-3-yl)-1,2,4-oxadiazol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186405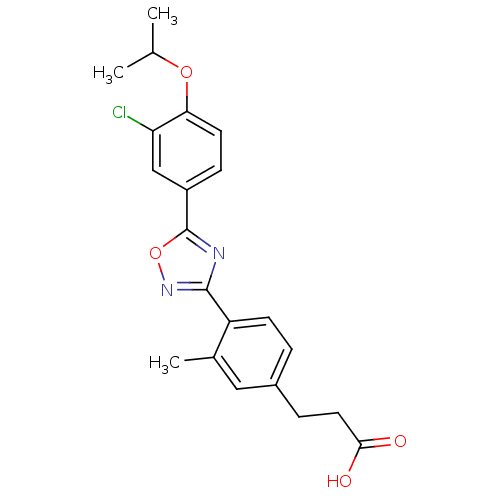 (3-(4-(5-(3-chloro-4-isopropoxyphenyl)-1,2,4-oxadia...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186930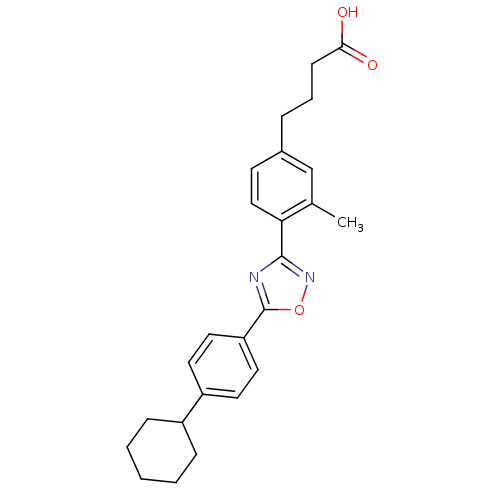 (4-(4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186927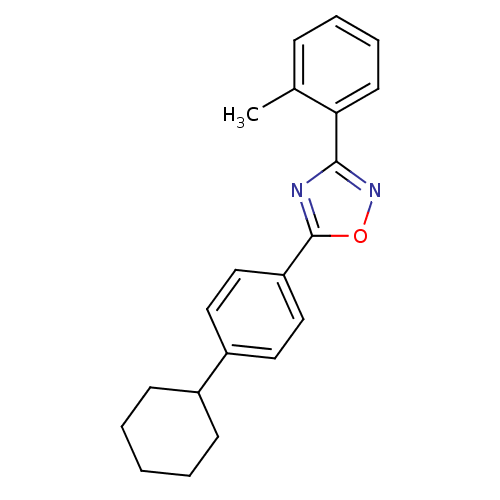 (5-(4-cyclohexylphenyl)-3-o-tolyl-1,2,4-oxadiazole ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 266 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186919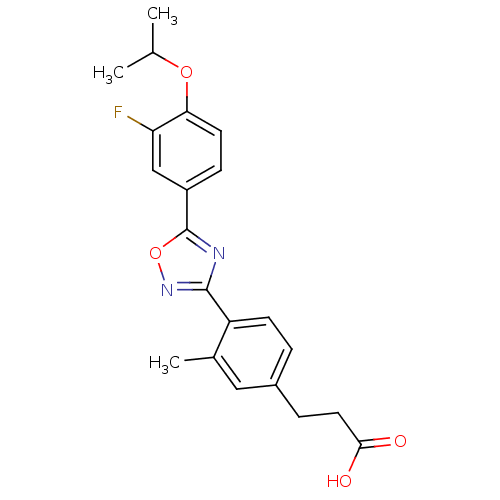 (3-(4-(5-(3-fluoro-4-isopropoxyphenyl)-1,2,4-oxadia...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186916 (3-(4-(5-(5-butylpyridin-2-yl)-1,2,4-oxadiazol-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50186915 (3-(4-(5-(6-isobutylpyridin-3-yl)-1,2,4-oxadiazol-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186926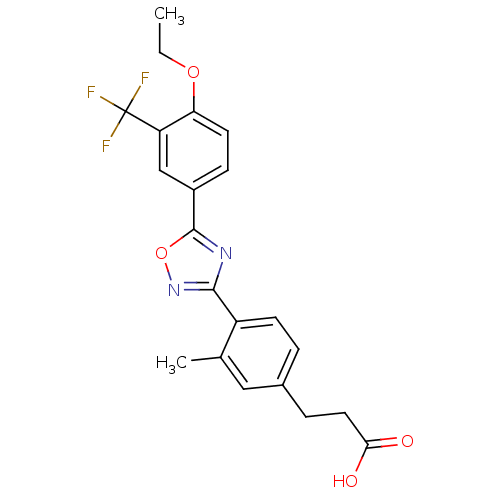 (3-(4-(5-(4-ethoxy-3-(trifluoromethyl)phenyl)-1,2,4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186935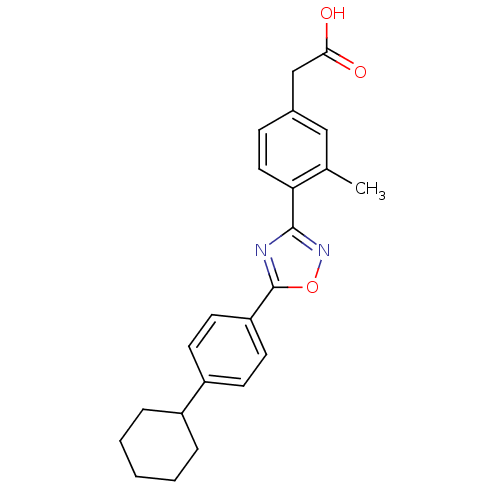 (2-(4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM22222 (3-(3-methyl-4-{5-[4-(propan-2-yloxy)-3-(trifluorom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50186931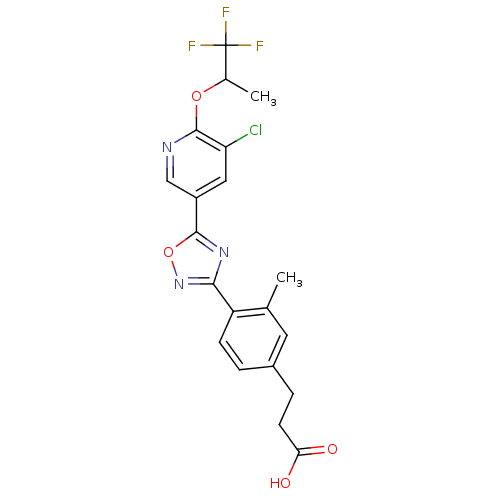 (3-(4-(5-(5-chloro-6-(1,1,1-trifluoropropan-2-yloxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186405 (3-(4-(5-(3-chloro-4-isopropoxyphenyl)-1,2,4-oxadia...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186923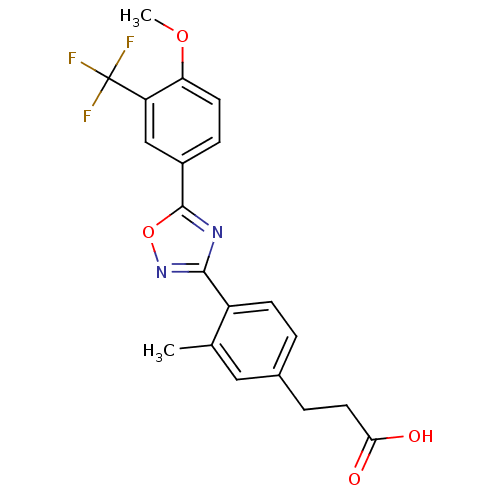 (3-(4-(5-(4-methoxy-3-(trifluoromethyl)phenyl)-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186931 (3-(4-(5-(5-chloro-6-(1,1,1-trifluoropropan-2-yloxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186923 (3-(4-(5-(4-methoxy-3-(trifluoromethyl)phenyl)-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM22222 (3-(3-methyl-4-{5-[4-(propan-2-yloxy)-3-(trifluorom...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186927 (5-(4-cyclohexylphenyl)-3-o-tolyl-1,2,4-oxadiazole ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50186920 (3-(4-(5-(3-bromo-4-isopropoxyphenyl)-1,2,4-oxadiaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186922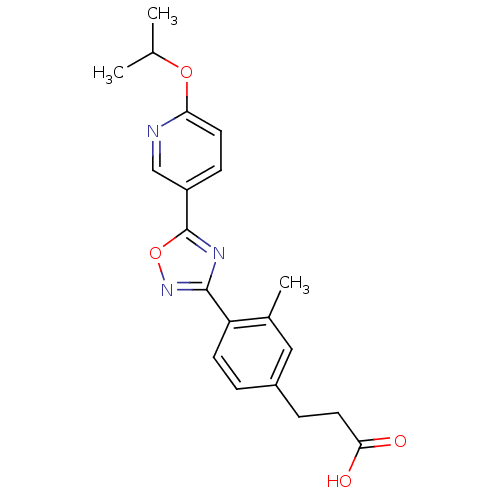 (3-(4-(5-(6-isopropoxypyridin-3-yl)-1,2,4-oxadiazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50186922 (3-(4-(5-(6-isopropoxypyridin-3-yl)-1,2,4-oxadiazol...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186404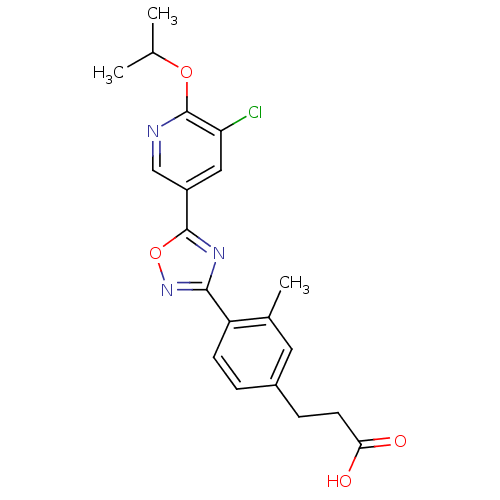 (3-(4-(5-(5-chloro-6-isopropoxypyridin-3-yl)-1,2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186936 (3-(4-(5-(3-cyano-4-(1,1,1-trifluoropropan-2-yloxy)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186918 (3-(4-(5-(4-(S)-sec-butoxy-3-cyanophenyl)-1,2,4-oxa...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186933 (3-(4-(5-(5-(1,1-difluoro-2-methylpropyl)pyridin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186928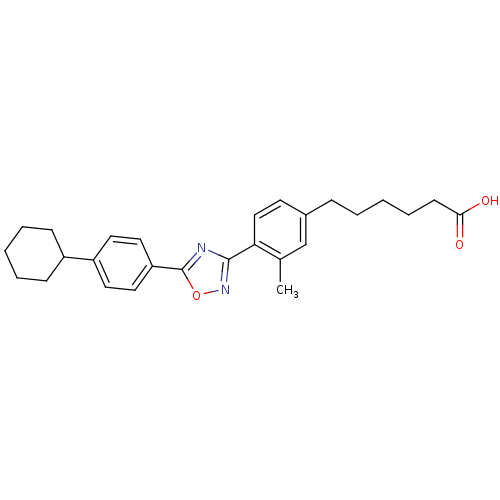 (6-(4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50186928 (6-(4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P1 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186924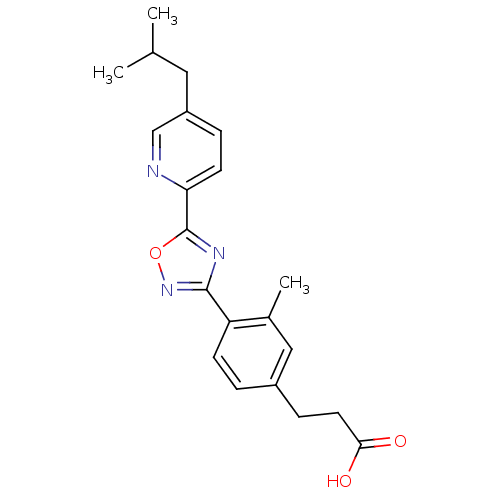 (3-(4-(5-(5-isobutylpyridin-2-yl)-1,2,4-oxadiazol-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186916 (3-(4-(5-(5-butylpyridin-2-yl)-1,2,4-oxadiazol-3-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186929 (3-(4-(5-(3-cyano-4-(1,1,1,3,3,3-hexafluoropropan-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22223 (3-(4-{5-[3-cyano-4-(propan-2-yloxy)phenyl]-1,2,4-o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186932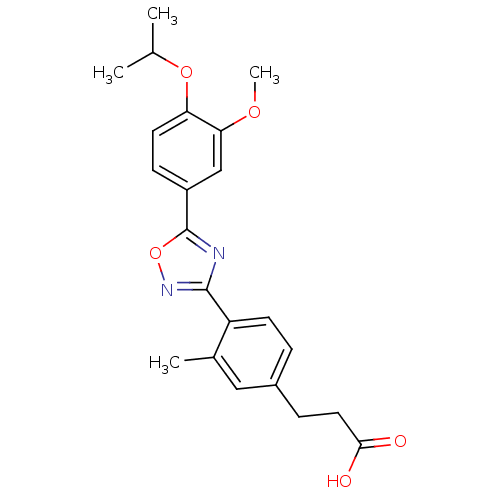 (3-(4-(5-(4-isopropoxy-3-methoxyphenyl)-1,2,4-oxadi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186925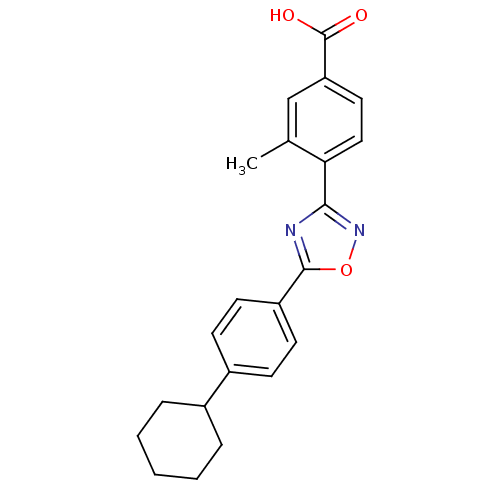 (4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186405 (3-(4-(5-(3-chloro-4-isopropoxyphenyl)-1,2,4-oxadia...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50186921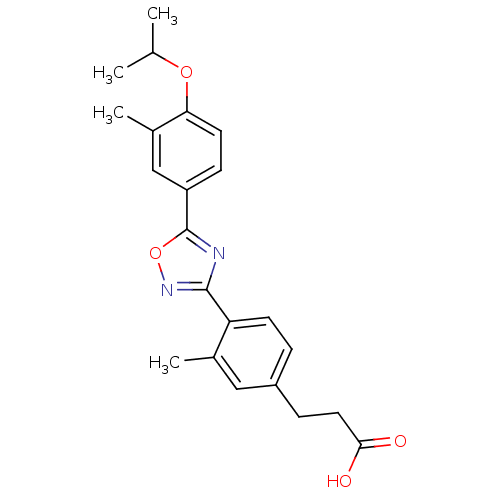 (3-(4-(5-(4-isopropoxy-3-methylphenyl)-1,2,4-oxadia...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P3 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50186925 (4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)-3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186930 (4-(4-(5-(4-cyclohexylphenyl)-1,2,4-oxadiazol-3-yl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50186405 (3-(4-(5-(3-chloro-4-isopropoxyphenyl)-1,2,4-oxadia...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P2 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186924 (3-(4-(5-(5-isobutylpyridin-2-yl)-1,2,4-oxadiazol-3...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186929 (3-(4-(5-(3-cyano-4-(1,1,1,3,3,3-hexafluoropropan-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186934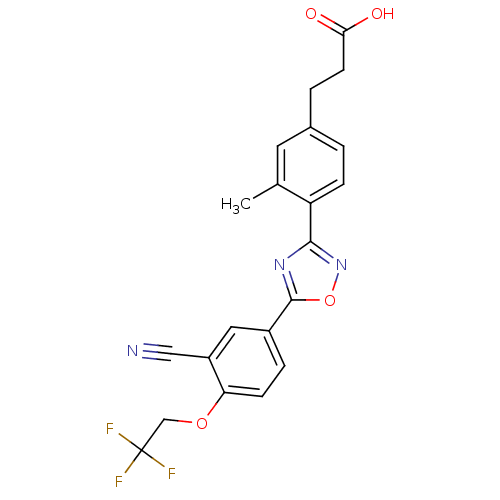 (3-(4-(5-(3-cyano-4-(2,2,2-trifluoroethoxy)phenyl)-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186915 (3-(4-(5-(6-isobutylpyridin-3-yl)-1,2,4-oxadiazol-3...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM22222 (3-(3-methyl-4-{5-[4-(propan-2-yloxy)-3-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186922 (3-(4-(5-(6-isopropoxypyridin-3-yl)-1,2,4-oxadiazol...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186920 (3-(4-(5-(3-bromo-4-isopropoxyphenyl)-1,2,4-oxadiaz...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50186923 (3-(4-(5-(4-methoxy-3-(trifluoromethyl)phenyl)-1,2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Activity at S1P4 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake | Bioorg Med Chem Lett 16: 3679-83 (2006) Article DOI: 10.1016/j.bmcl.2006.04.084 BindingDB Entry DOI: 10.7270/Q2J67GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131 total ) | Next | Last >> |