

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090760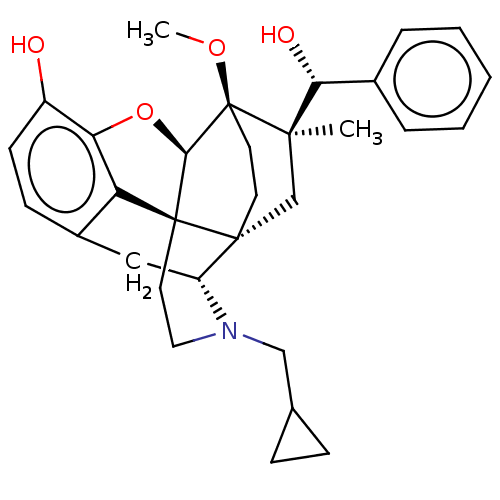 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090731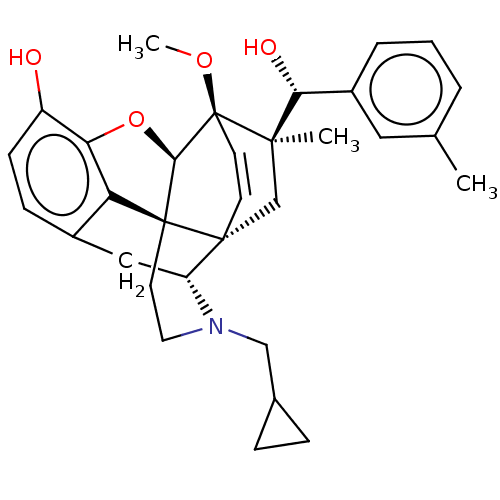 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50015003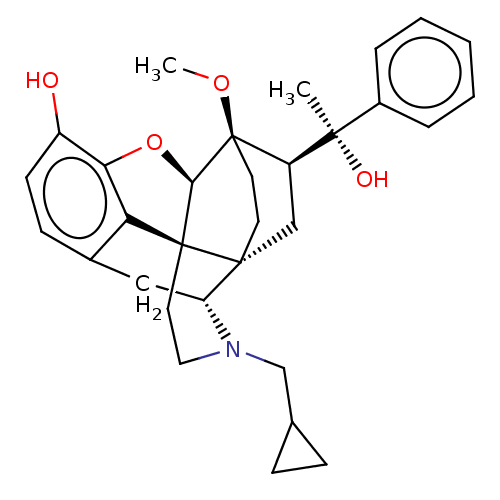 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090755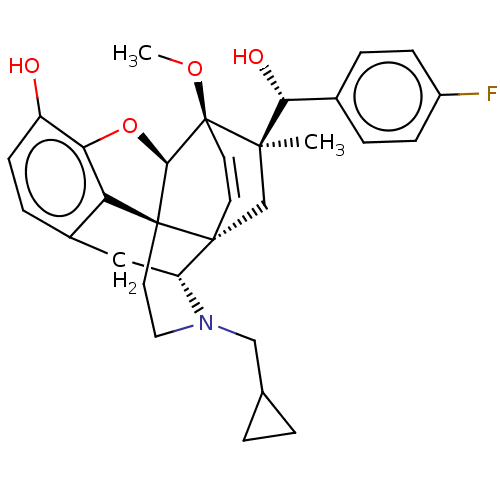 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090766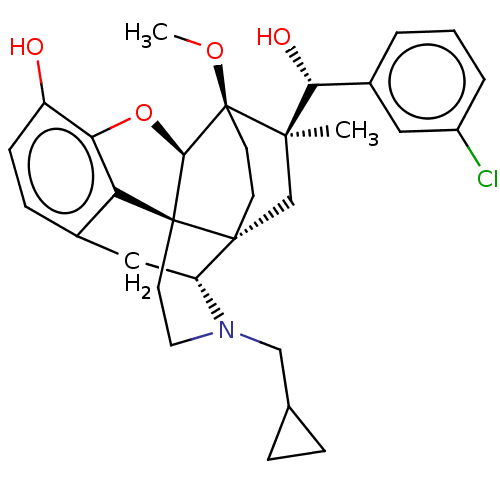 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090782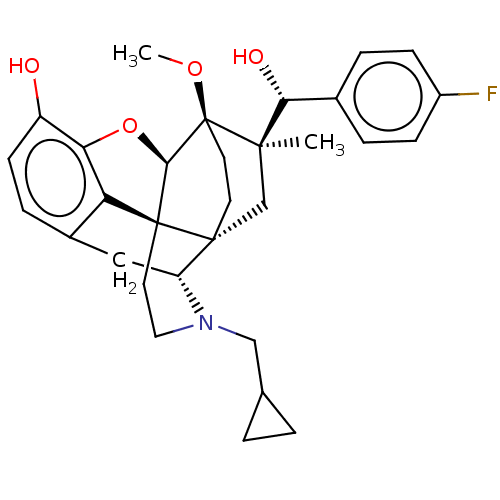 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090694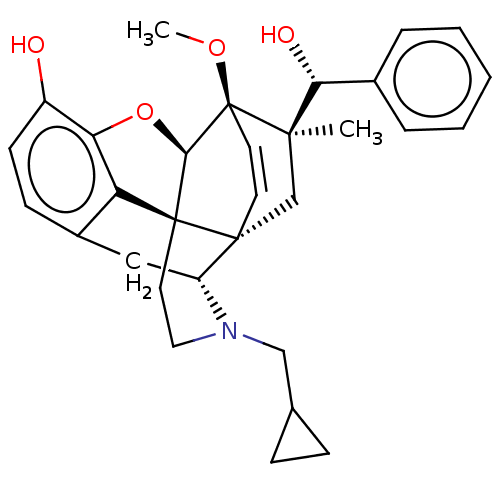 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090694 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090766 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090765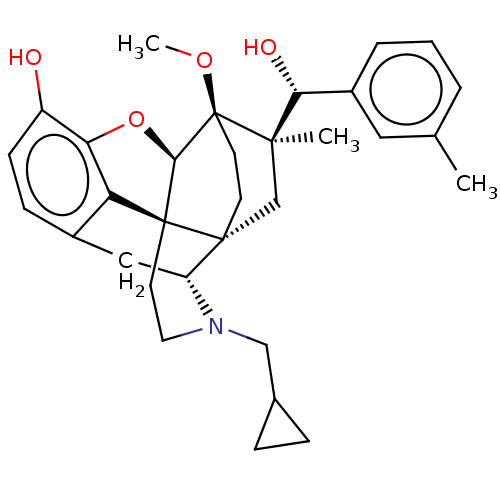 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090782 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090768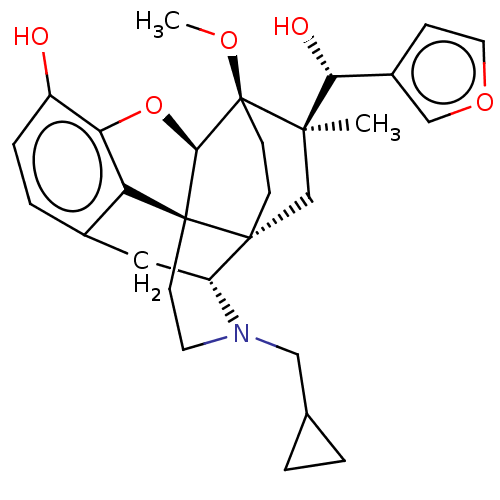 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090765 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090769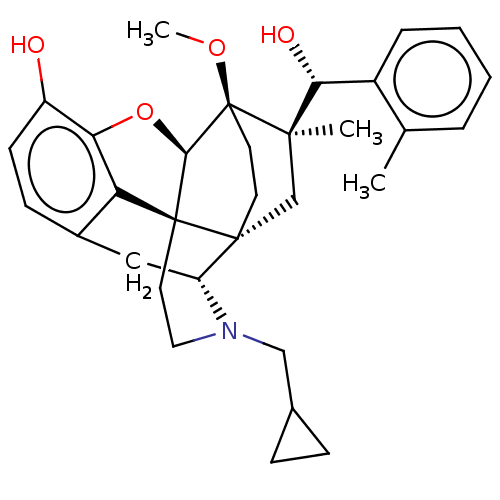 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090773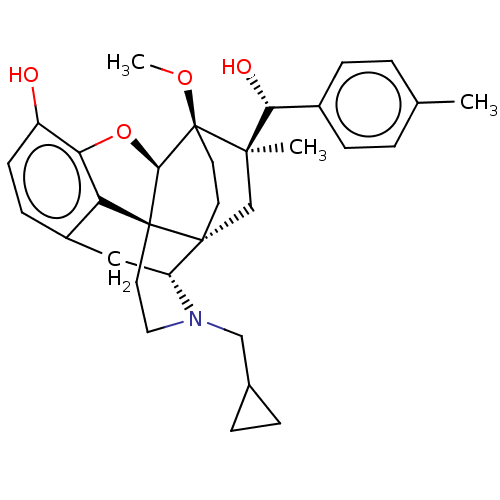 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090768 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50090773 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090755 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090769 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090782 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090694 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090903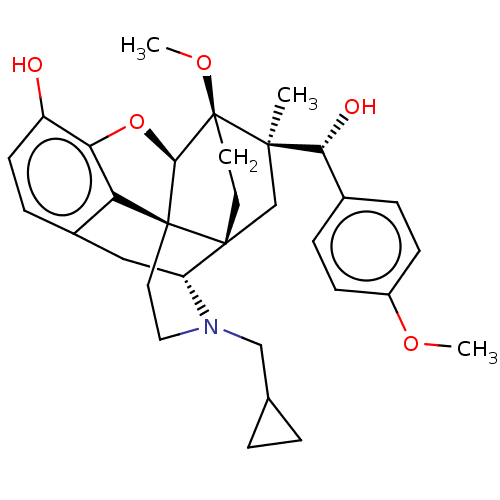 (CHEMBL3581755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090755 (CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50015003 (CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090904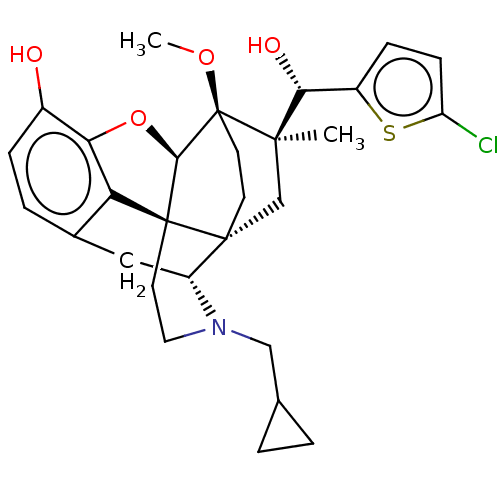 (CHEMBL3581760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090766 (CHEMBL3581756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090773 (CHEMBL3581753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090782 (CHEMBL3581754) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090767 (CHEMBL3581757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090760 (CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090694 (CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090765 (CHEMBL3581752) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090905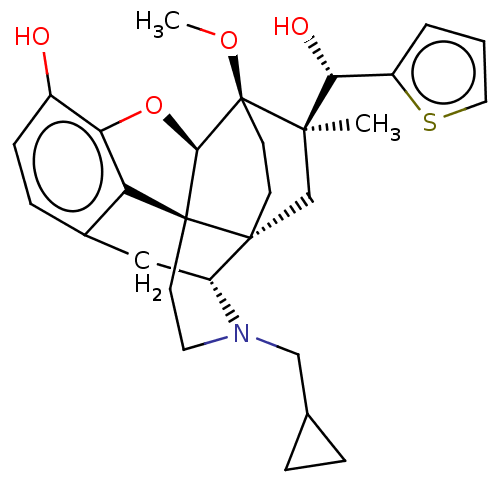 (CHEMBL3581761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 571 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090769 (CHEMBL3581751) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090902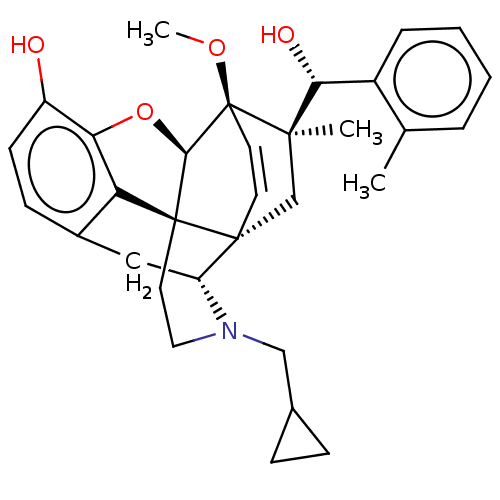 (CHEMBL3580675) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50090768 (CHEMBL3581762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in HEK293 cell membranes incubated for 1 hr by beta counting method | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090731 (CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50090906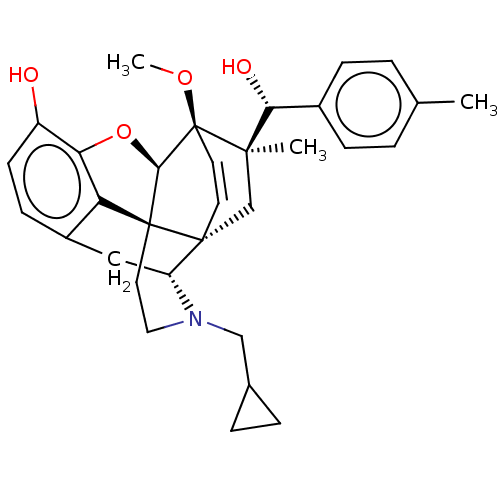 (CHEMBL3581742) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in rat C6 cell membranes assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 58: 4242-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00130 BindingDB Entry DOI: 10.7270/Q2ST7RKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |