

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194208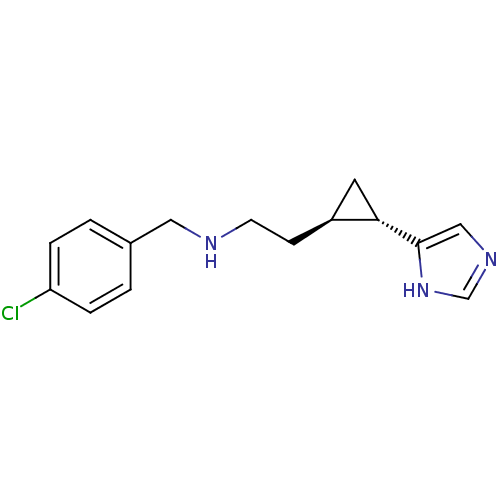 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194202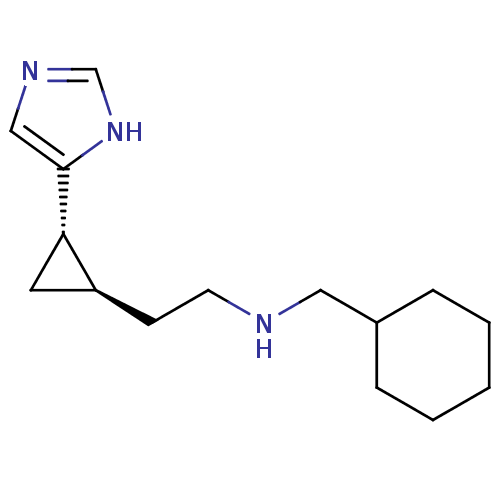 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194205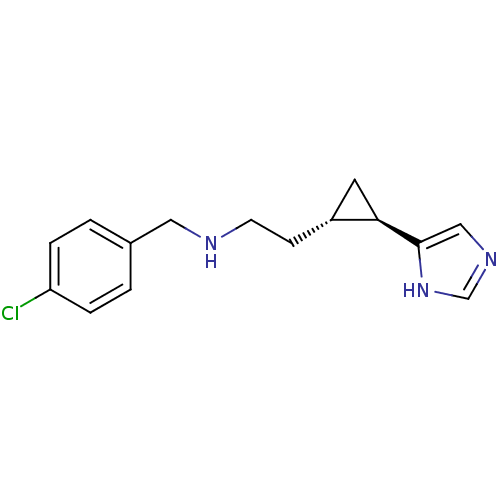 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194205 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194203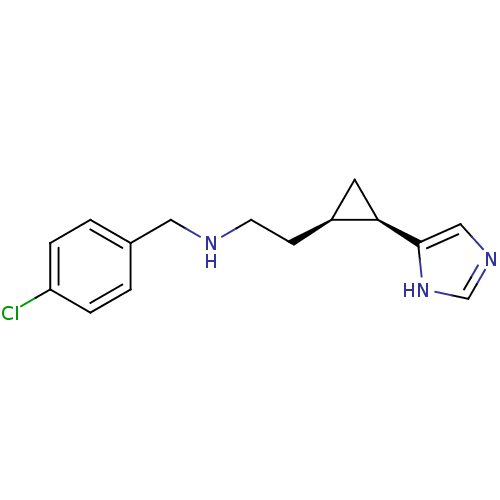 ((1R,2R)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194211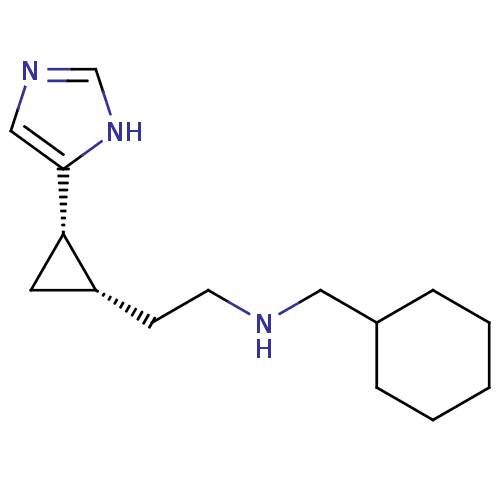 ((1S,2S)-cis-2-[2-(cyclohexylmethylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194206 ((1R,2S)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194209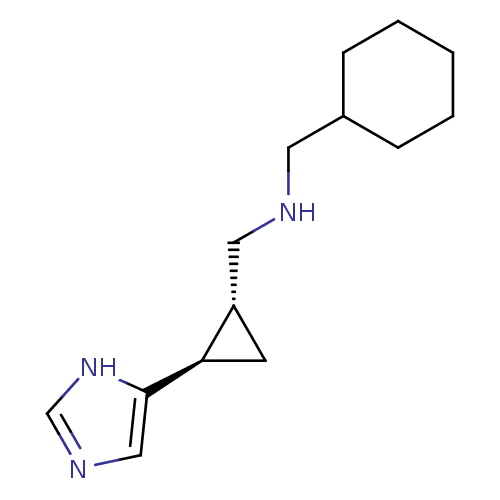 ((1R,2R)-trans-2-(cyclohexylmethylamino)methyl-1-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194206 ((1R,2S)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194208 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194212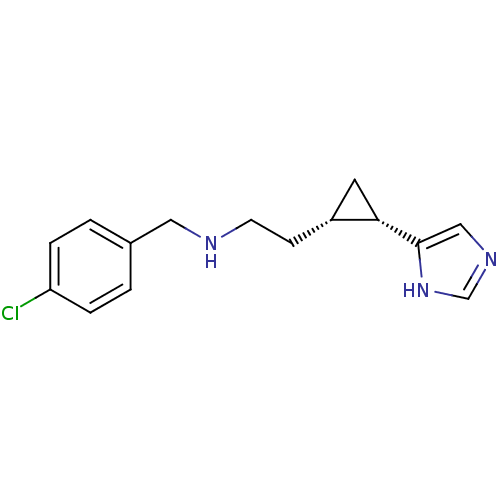 ((1S,2S)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 51.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194204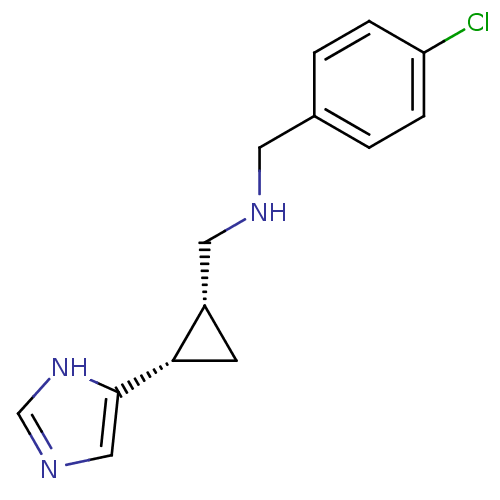 ((1S,2R)-cis-2-(4-chlorobenzylamino)methyl-1-(1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194216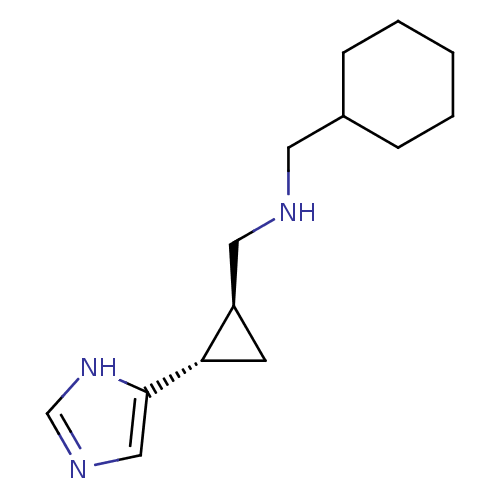 ((1S,2S)-trans-2-(cyclohexylmethylamino)methyl-1-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194203 ((1R,2R)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194210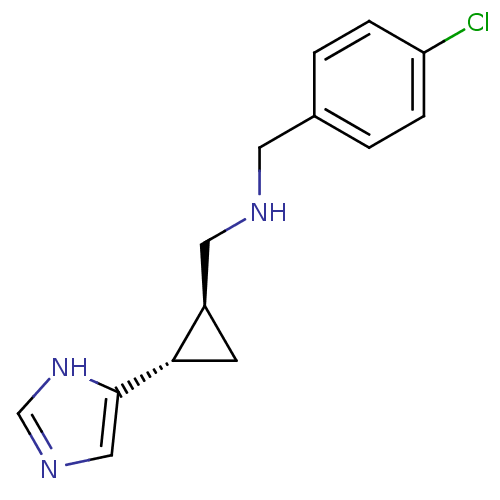 ((1S,2S)-trans-2-(4-chlorobenzylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194207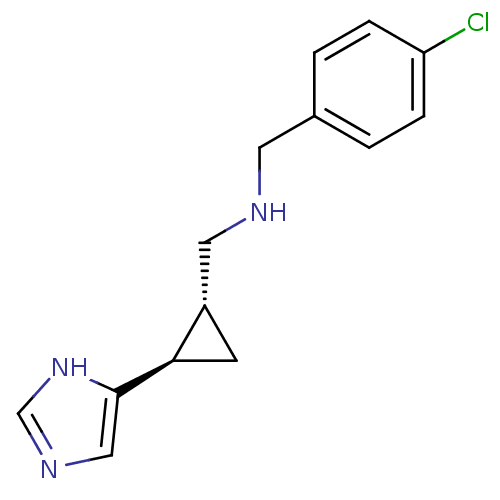 ((1R,2R)-trans-2-(4-chlorobenzylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194212 ((1S,2S)-cis-2-[2-(4-chlorobenzylamino)ethyl]-1-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194209 ((1R,2R)-trans-2-(cyclohexylmethylamino)methyl-1-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194211 ((1S,2S)-cis-2-[2-(cyclohexylmethylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194210 ((1S,2S)-trans-2-(4-chlorobenzylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194216 ((1S,2S)-trans-2-(cyclohexylmethylamino)methyl-1-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 289 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194215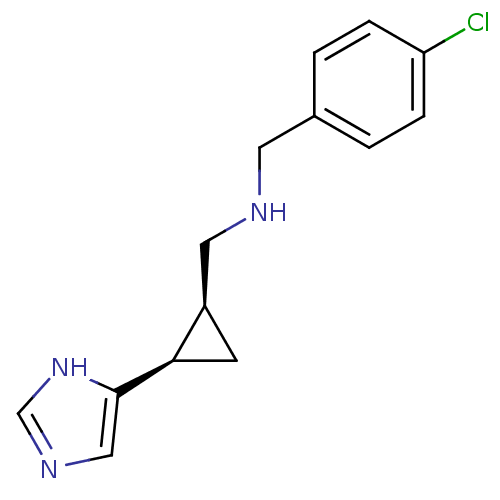 ((1R,2S)-cis-2-(4-chlorobenzylamino)methyl-1-(1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194217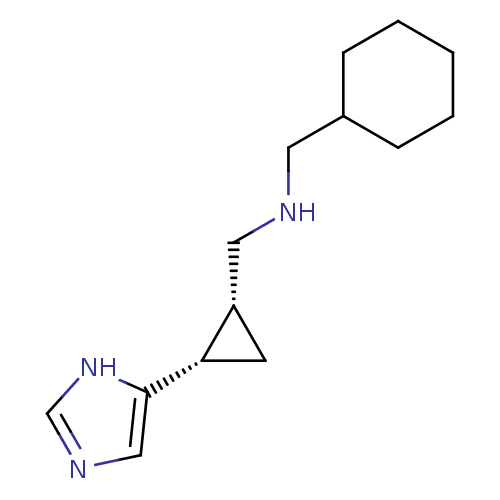 ((1S,2R)-cis-2-(cyclohexylmethylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194214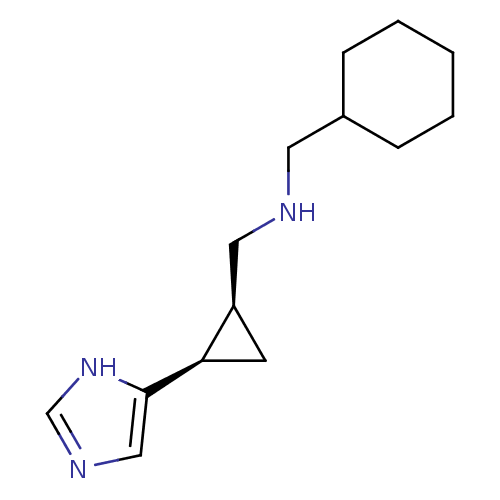 ((1R,2S)-cis-2-(cyclohexylmethylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194207 ((1R,2R)-trans-2-(4-chlorobenzylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194213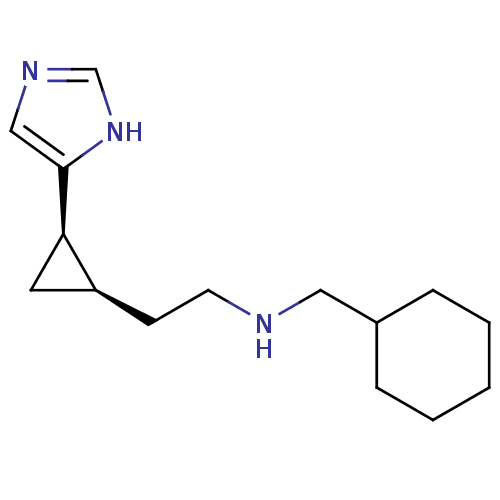 ((1R,2R)-cis-2-[2-(cyclohexylmethylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194215 ((1R,2S)-cis-2-(4-chlorobenzylamino)methyl-1-(1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194213 ((1R,2R)-cis-2-[2-(cyclohexylmethylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194214 ((1R,2S)-cis-2-(cyclohexylmethylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194204 ((1S,2R)-cis-2-(4-chlorobenzylamino)methyl-1-(1H-im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]histamine form human H4 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194217 ((1S,2R)-cis-2-(cyclohexylmethylamino)methyl-1-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Displacement of [3H]Nalpha-methylhistamine form human H3 receptor | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194208 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194205 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H3 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194205 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194208 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H4 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50194205 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50194208 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50194208 ((1S,2R)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H2 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H2 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50194205 ((1R,2S)-trans-2-[2-(4-chlorobenzylamino)ethyl]-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H2 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Antagonist activity at human H1 receptor expressed in 293-EBNA cells assessed as inhibition of histamine agonist activity by luciferase reporter gene... | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonist activity at human H1 receptor expressed in 293-EBNA cells by luciferase reporter gene assay | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50194202 ((1S,2R)-trans-2-[2-(cyclohexylmethylamino)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonist activity at human H1 receptor expressed in 293-EBNA cells by luciferase reporter gene assay | J Med Chem 49: 5587-96 (2006) Article DOI: 10.1021/jm0603318 BindingDB Entry DOI: 10.7270/Q2MW2GRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 64 total ) | Next | Last >> |