

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089653 (CHEMBL3576974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089655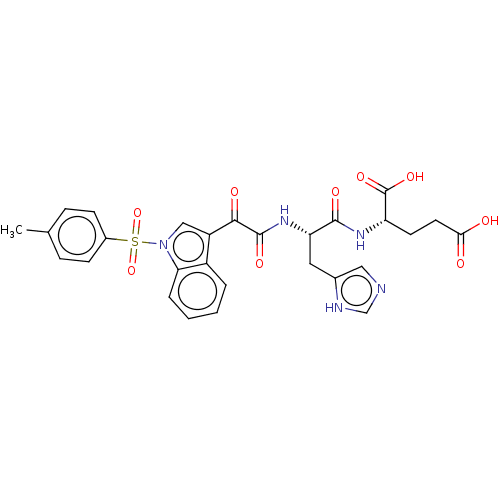 (CHEMBL3576976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089651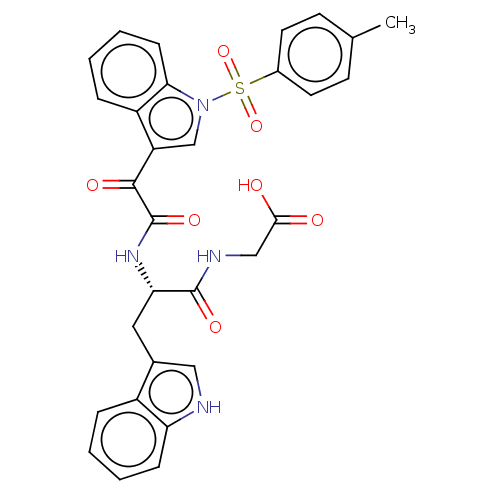 (CHEMBL3576979) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089648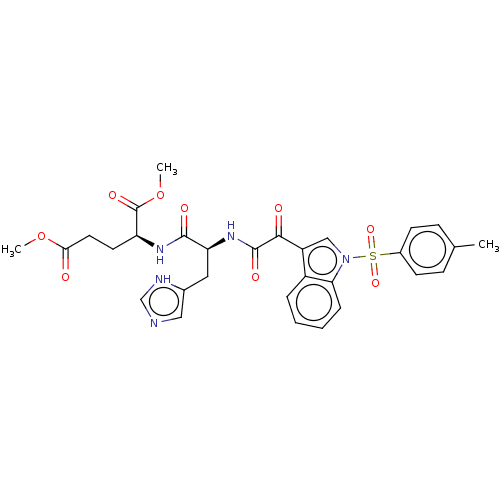 (CHEMBL3576983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 983 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089646 (CHEMBL3576981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089644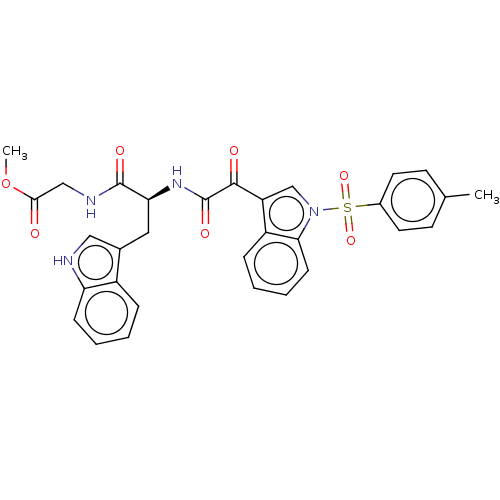 (CHEMBL3576986) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089656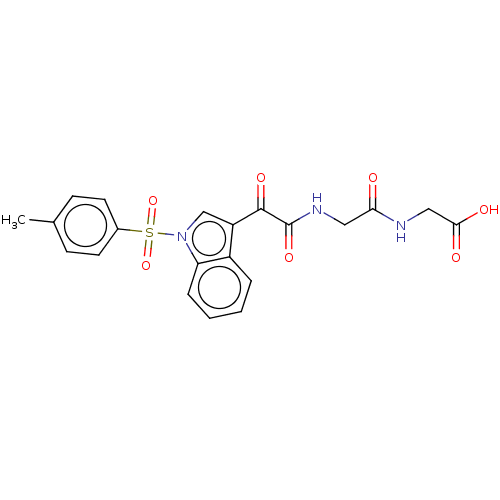 (CHEMBL3576973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089649 (CHEMBL3576977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant COX-2 by UV-Visible spectrophotometry | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089651 (CHEMBL3576979) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089644 (CHEMBL3576986) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50096276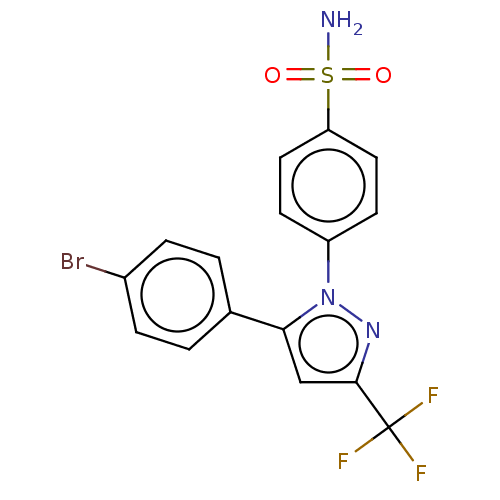 (CHEMBL1235806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089653 (CHEMBL3576974) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089655 (CHEMBL3576976) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM13066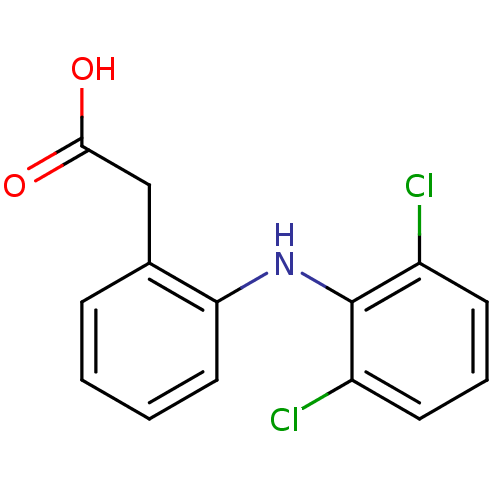 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13066 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089656 (CHEMBL3576973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089642 (CHEMBL3576984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089650 (CHEMBL3576978) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089651 (CHEMBL3576979) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089652 (CHEMBL3576980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089648 (CHEMBL3576983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089645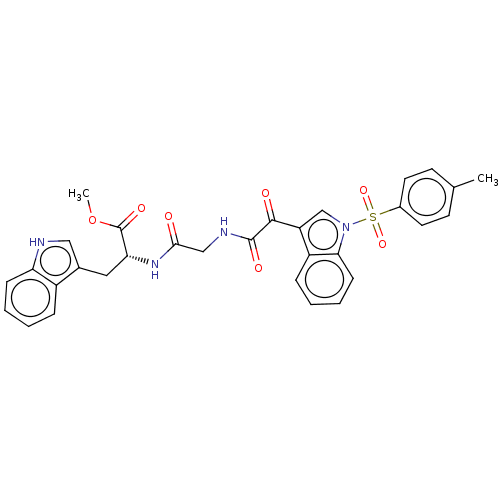 (CHEMBL3576987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089647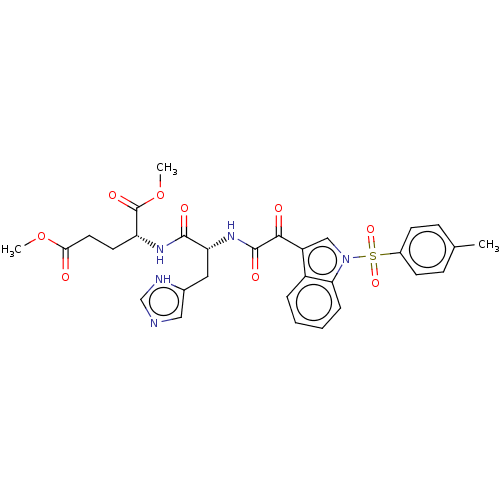 (CHEMBL3576982) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089646 (CHEMBL3576981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50446681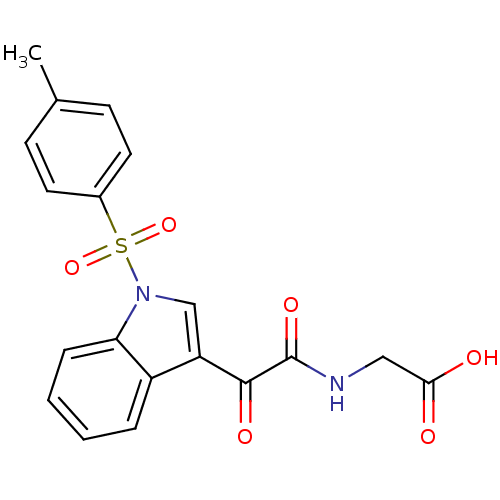 (CHEMBL3113617) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089644 (CHEMBL3576986) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089643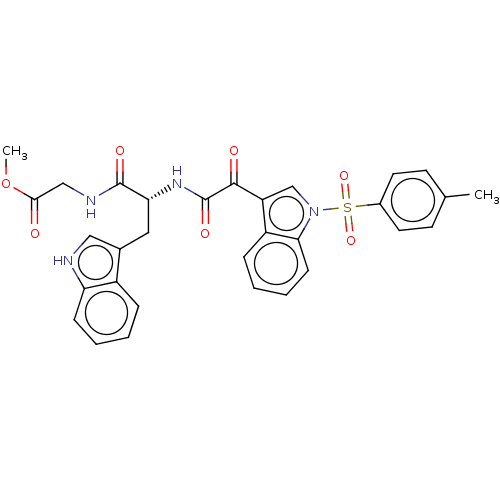 (CHEMBL3576985) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089649 (CHEMBL3576977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089655 (CHEMBL3576976) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50096276 (CHEMBL1235806) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089641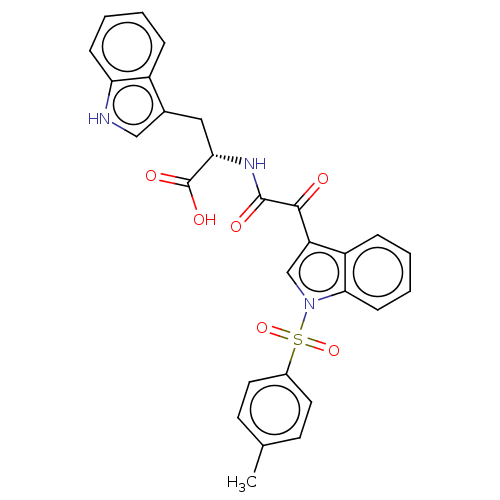 (CHEMBL3576988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089647 (CHEMBL3576982) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50089654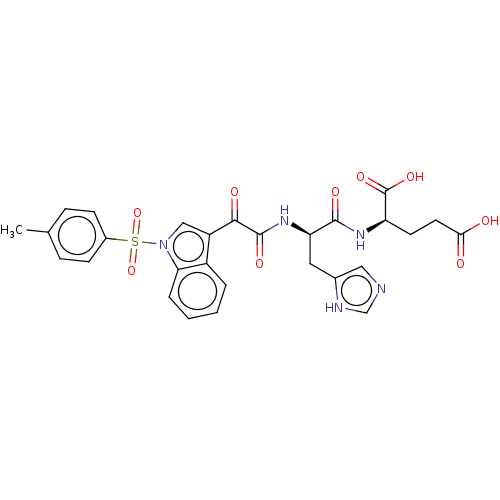 (CHEMBL3576975) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089653 (CHEMBL3576974) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50140257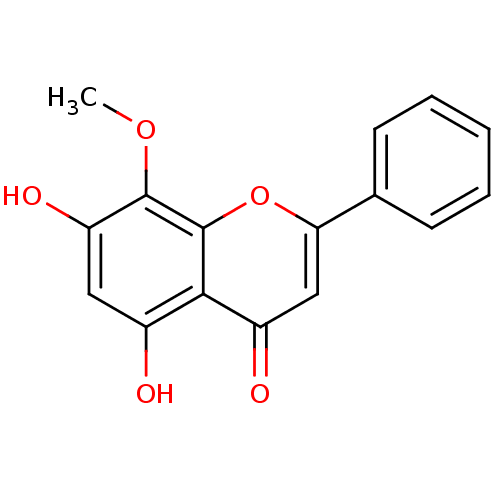 (5,7-Dihydroxy-8-methoxy-2-phenyl-chromen-4-one | 5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 assessed as PGF2 alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089645 (CHEMBL3576987) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089643 (CHEMBL3576985) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089648 (CHEMBL3576983) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089641 (CHEMBL3576988) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50446681 (CHEMBL3113617) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089646 (CHEMBL3576981) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089652 (CHEMBL3576980) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089642 (CHEMBL3576984) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089656 (CHEMBL3576973) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089650 (CHEMBL3576978) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50089649 (CHEMBL3576977) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as PGF2alpha formation using arachidonic acid as substrate pretreated with compound for 20 mins prior to substrate... | Eur J Med Chem 97: 104-23 (2015) Article DOI: 10.1016/j.ejmech.2015.04.044 BindingDB Entry DOI: 10.7270/Q2N87CJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 51 total ) | Next | Last >> |