 Found 75 hits Enz. Inhib. hit(s) with all data for entry = 50046284
Found 75 hits Enz. Inhib. hit(s) with all data for entry = 50046284 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50103543
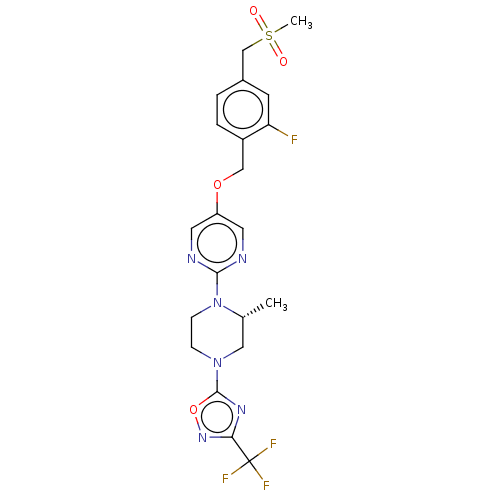
(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103561
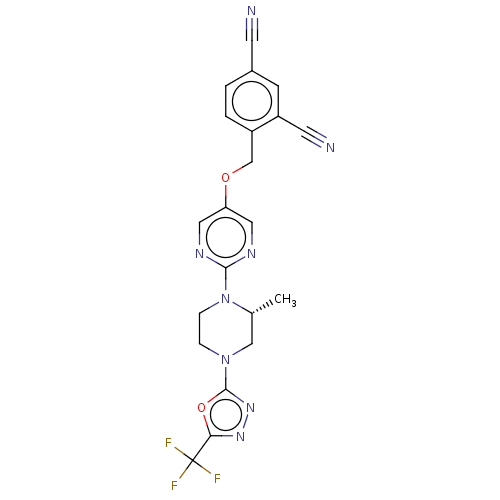
(CHEMBL3357996)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2C#N)C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H17F3N8O2/c1-13-11-31(20-30-29-18(34-20)21(22,23)24)4-5-32(13)19-27-9-17(10-28-19)33-12-15-3-2-14(7-25)6-16(15)8-26/h2-3,6,9-10,13H,4-5,11-12H2,1H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103544
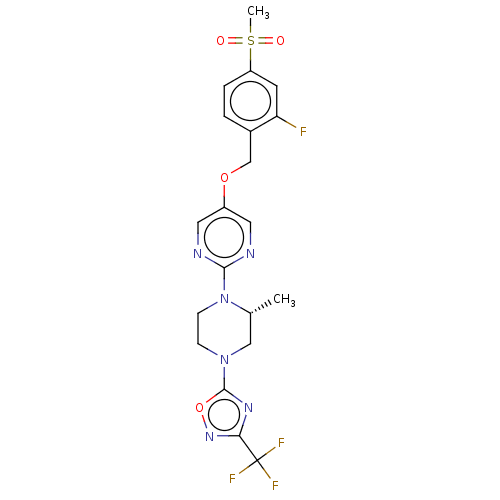
(CHEMBL3358005)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O4S/c1-12-10-29(19-27-17(28-34-19)20(22,23)24)5-6-30(12)18-25-8-14(9-26-18)33-11-13-3-4-15(7-16(13)21)35(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103557
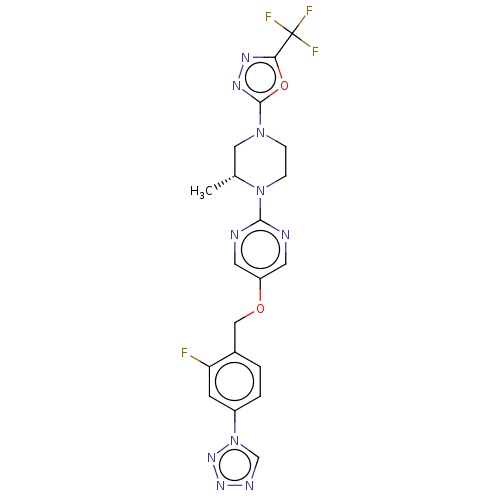
(CHEMBL3358000)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)-n2cnnn2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F4N10O2/c1-12-9-32(19-29-28-17(36-19)20(22,23)24)4-5-33(12)18-25-7-15(8-26-18)35-10-13-2-3-14(6-16(13)21)34-11-27-30-31-34/h2-3,6-8,11-12H,4-5,9-10H2,1H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103562
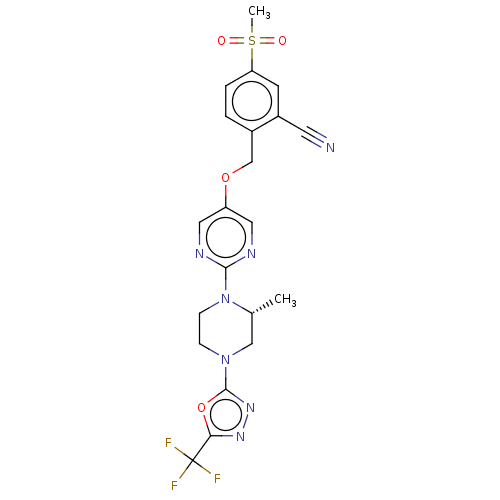
(CHEMBL3357995)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2C#N)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N7O4S/c1-13-11-30(20-29-28-18(35-20)21(22,23)24)5-6-31(13)19-26-9-16(10-27-19)34-12-14-3-4-17(36(2,32)33)7-15(14)8-25/h3-4,7,9-10,13H,5-6,11-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103563

(CHEMBL3357994)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O4S/c1-12-10-29(19-28-27-17(34-19)20(22,23)24)5-6-30(12)18-25-8-14(9-26-18)33-11-13-3-4-15(7-16(13)21)35(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103552

(CHEMBL3358014)Show SMILES CC(C)c1noc(n1)N1CCN([C@H](C)C1)c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1 |r| Show InChI InChI=1S/C22H27FN6O4S/c1-14(2)20-26-22(33-27-20)28-7-8-29(15(3)12-28)21-24-10-17(11-25-21)32-13-16-5-6-18(9-19(16)23)34(4,30)31/h5-6,9-11,14-15H,7-8,12-13H2,1-4H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103560
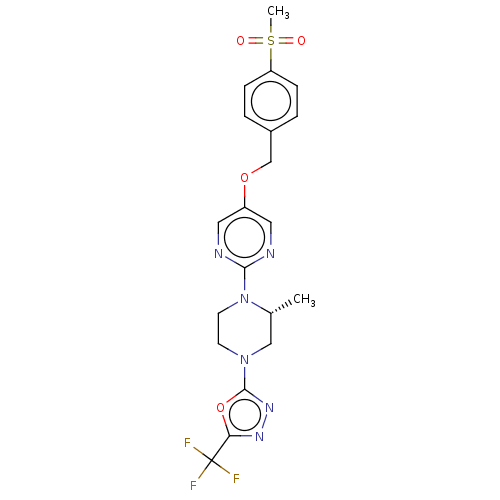
(CHEMBL3357997)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H21F3N6O4S/c1-13-11-28(19-27-26-17(33-19)20(21,22)23)7-8-29(13)18-24-9-15(10-25-18)32-12-14-3-5-16(6-4-14)34(2,30)31/h3-6,9-10,13H,7-8,11-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by high throughput fluorescence assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) by high throughput fluorescence assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by high throughput fluorescence assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by high throughput fluorescence assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) by high throughput fluorescence assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103550

(CHEMBL3358012)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1)C(=O)OC1(COC1)C(F)(F)F |r| Show InChI InChI=1S/C22H24F4N4O6S/c1-14-10-29(20(31)36-21(12-34-13-21)22(24,25)26)5-6-30(14)19-27-8-16(9-28-19)35-11-15-3-4-17(7-18(15)23)37(2,32)33/h3-4,7-9,14H,5-6,10-13H2,1-2H3/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103558
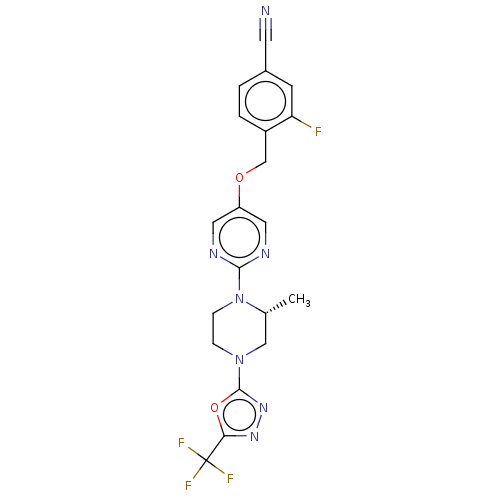
(CHEMBL3357999)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H17F4N7O2/c1-12-10-30(19-29-28-17(33-19)20(22,23)24)4-5-31(12)18-26-8-15(9-27-18)32-11-14-3-2-13(7-25)6-16(14)21/h2-3,6,8-9,12H,4-5,10-11H2,1H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103555

(CHEMBL3358002)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(OS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O5S/c1-12-10-29(19-28-27-17(34-19)20(22,23)24)5-6-30(12)18-25-8-15(9-26-18)33-11-13-3-4-14(7-16(13)21)35-36(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103564
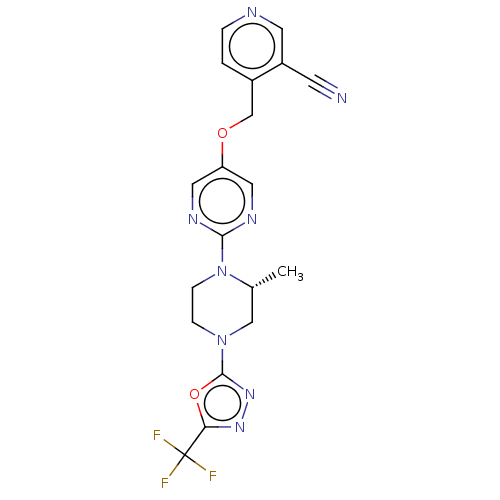
(CHEMBL3218816)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C19H17F3N8O2/c1-12-10-29(18-28-27-16(32-18)19(20,21)22)4-5-30(12)17-25-8-15(9-26-17)31-11-13-2-3-24-7-14(13)6-23/h2-3,7-9,12H,4-5,10-11H2,1H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily E member 1
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IKs channel (unknown origin) |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103545
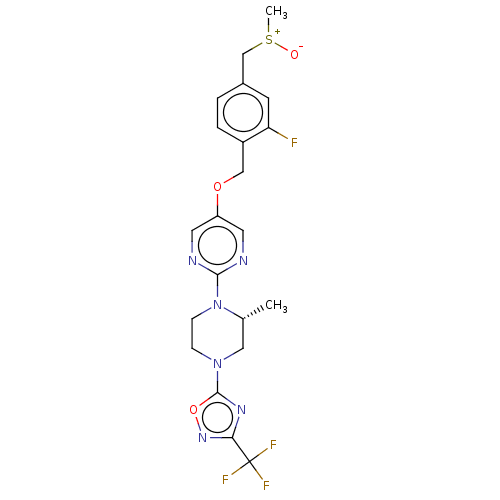
(CHEMBL3358007)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(C[S+](C)[O-])cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O3S/c1-13-10-30(20-28-18(29-34-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)33-11-15-4-3-14(7-17(15)22)12-35(2)32/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103556
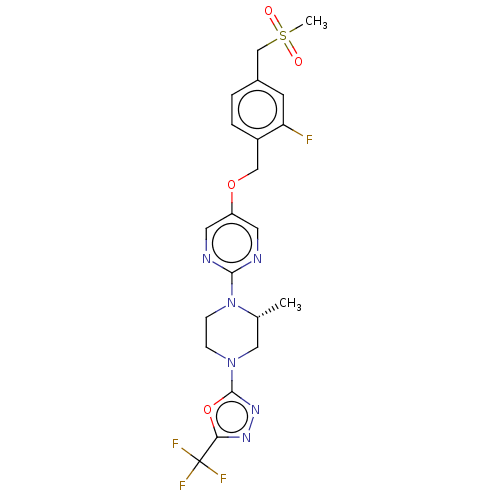
(CHEMBL3358001)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-29-28-18(35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103559

(CHEMBL3357998)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F3N6O4S/c1-14-11-29(20-28-27-18(34-20)21(22,23)24)7-8-30(14)19-25-9-17(10-26-19)33-12-15-3-5-16(6-4-15)13-35(2,31)32/h3-6,9-10,14H,7-8,11-13H2,1-2H3/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103551

(CHEMBL3358013)Show SMILES CC(C)c1noc(n1)N1CCN([C@H](C)C1)c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1 |r| Show InChI InChI=1S/C23H29FN6O4S/c1-15(2)21-27-23(34-28-21)29-7-8-30(16(3)12-29)22-25-10-19(11-26-22)33-13-18-6-5-17(9-20(18)24)14-35(4,31)32/h5-6,9-11,15-16H,7-8,12-14H2,1-4H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103549
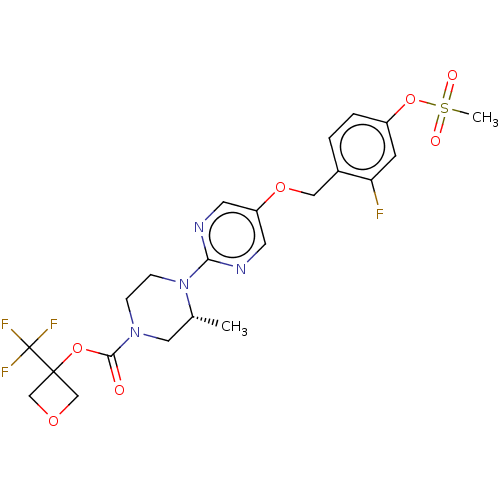
(CHEMBL3358011)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(OS(C)(=O)=O)cc2F)cn1)C(=O)OC1(COC1)C(F)(F)F |r| Show InChI InChI=1S/C22H24F4N4O7S/c1-14-10-29(20(31)36-21(12-34-13-21)22(24,25)26)5-6-30(14)19-27-8-17(9-28-19)35-11-15-3-4-16(7-18(15)23)37-38(2,32)33/h3-4,7-9,14H,5-6,10-13H2,1-2H3/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103548

(CHEMBL3358010)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)C(=O)OC1(COC1)C(F)(F)F |r| Show InChI InChI=1S/C23H26F4N4O6S/c1-15-10-30(21(32)37-22(13-35-14-22)23(25,26)27)5-6-31(15)20-28-8-18(9-29-20)36-11-17-4-3-16(7-19(17)24)12-38(2,33)34/h3-4,7-9,15H,5-6,10-14H2,1-2H3/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103554
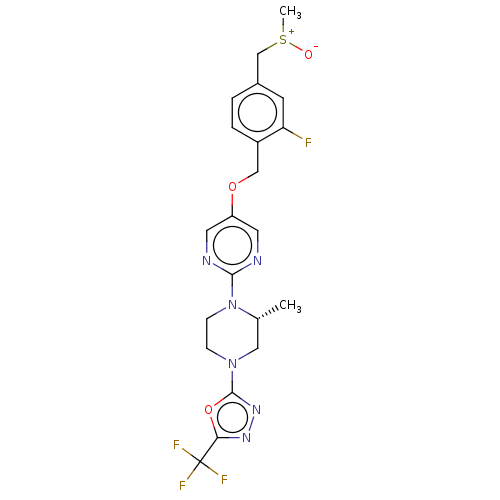
(CHEMBL3358003)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(C[S+](C)[O-])cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O3S/c1-13-10-30(20-29-28-18(34-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)33-11-15-4-3-14(7-17(15)22)12-35(2)32/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103553

(CHEMBL3358004)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=N)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F4N7O3S/c1-13-10-31(20-30-29-18(35-20)21(23,24)25)5-6-32(13)19-27-8-16(9-28-19)34-11-15-4-3-14(7-17(15)22)12-36(2,26)33/h3-4,7-9,13,26H,5-6,10-12H2,1-2H3/t13-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NaV1.5 (unknown origin) |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1C
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CaV1.2 (unknown origin) |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103547
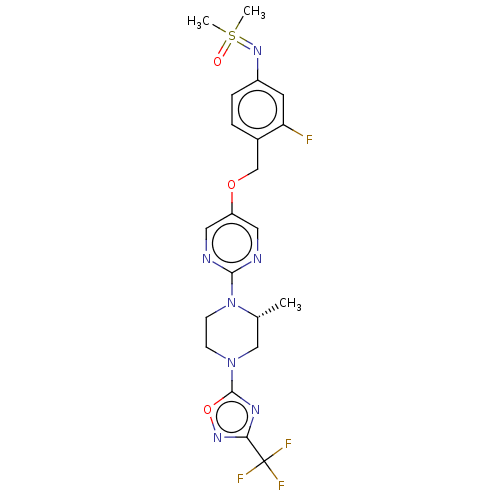
(CHEMBL3358009)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)N=S(C)(C)=O)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F4N7O3S/c1-13-11-31(20-28-18(29-35-20)21(23,24)25)6-7-32(13)19-26-9-16(10-27-19)34-12-14-4-5-15(8-17(14)22)30-36(2,3)33/h4-5,8-10,13H,6-7,11-12H2,1-3H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103546
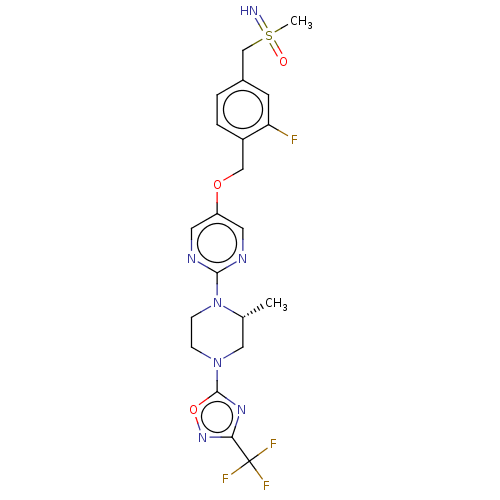
(CHEMBL3358008)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=N)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F4N7O3S/c1-13-10-31(20-29-18(30-35-20)21(23,24)25)5-6-32(13)19-27-8-16(9-28-19)34-11-15-4-3-14(7-17(15)22)12-36(2,26)33/h3-4,7-9,13,26H,5-6,10-12H2,1-2H3/t13-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103543

(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103553

(CHEMBL3358004)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=N)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F4N7O3S/c1-13-10-31(20-30-29-18(35-20)21(23,24)25)5-6-32(13)19-27-8-16(9-28-19)34-11-15-4-3-14(7-17(15)22)12-36(2,26)33/h3-4,7-9,13,26H,5-6,10-12H2,1-2H3/t13-,36?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103554

(CHEMBL3358003)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(C[S+](C)[O-])cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O3S/c1-13-10-30(20-29-28-18(34-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)33-11-15-4-3-14(7-17(15)22)12-35(2)32/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-,35?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 976 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103555

(CHEMBL3358002)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(OS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O5S/c1-12-10-29(19-28-27-17(34-19)20(22,23)24)5-6-30(12)18-25-8-15(9-26-18)33-11-13-3-4-14(7-16(13)21)35-36(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103556

(CHEMBL3358001)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-29-28-18(35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 249 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103557

(CHEMBL3358000)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)-n2cnnn2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F4N10O2/c1-12-9-32(19-29-28-17(36-19)20(22,23)24)4-5-33(12)18-25-7-15(8-26-18)35-10-13-2-3-14(6-16(13)21)34-11-27-30-31-34/h2-3,6-8,11-12H,4-5,9-10H2,1H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103558

(CHEMBL3357999)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H17F4N7O2/c1-12-10-30(19-29-28-17(33-19)20(22,23)24)4-5-31(12)18-26-8-15(9-27-18)32-11-14-3-2-13(7-25)6-16(14)21/h2-3,6,8-9,12H,4-5,10-11H2,1H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 216 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103559

(CHEMBL3357998)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F3N6O4S/c1-14-11-29(20-28-27-18(34-20)21(22,23)24)7-8-30(14)19-25-9-17(10-26-19)33-12-15-3-5-16(6-4-15)13-35(2,31)32/h3-6,9-10,14H,7-8,11-13H2,1-2H3/t14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 319 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103560

(CHEMBL3357997)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H21F3N6O4S/c1-13-11-28(19-27-26-17(33-19)20(21,22)23)7-8-29(13)18-24-9-15(10-25-18)32-12-14-3-5-16(6-4-14)34(2,30)31/h3-6,9-10,13H,7-8,11-12H2,1-2H3/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 297 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103562

(CHEMBL3357995)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2C#N)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N7O4S/c1-13-11-30(20-29-28-18(35-20)21(22,23)24)5-6-31(13)19-26-9-16(10-27-19)34-12-14-3-4-17(36(2,32)33)7-15(14)8-25/h3-4,7,9-10,13H,5-6,11-12H2,1-2H3/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103563

(CHEMBL3357994)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O4S/c1-12-10-29(19-28-27-17(34-19)20(22,23)24)5-6-30(12)18-25-8-14(9-26-18)33-11-13-3-4-15(7-16(13)21)35(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 144 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50103564

(CHEMBL3218816)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C19H17F3N8O2/c1-12-10-29(18-28-27-16(32-18)19(20,21)22)4-5-30(12)17-25-8-15(9-26-17)31-11-13-2-3-24-7-14(13)6-23/h2-3,7-9,12H,4-5,10-11H2,1H3/t12-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 436 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at mouse GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103553

(CHEMBL3358004)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=N)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F4N7O3S/c1-13-10-31(20-30-29-18(35-20)21(23,24)25)5-6-32(13)19-27-8-16(9-28-19)34-11-15-4-3-14(7-17(15)22)12-36(2,26)33/h3-4,7-9,13,26H,5-6,10-12H2,1-2H3/t13-,36?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103554

(CHEMBL3358003)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(C[S+](C)[O-])cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O3S/c1-13-10-30(20-29-28-18(34-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)33-11-15-4-3-14(7-17(15)22)12-35(2)32/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-,35?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103555

(CHEMBL3358002)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(OS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O5S/c1-12-10-29(19-28-27-17(34-19)20(22,23)24)5-6-30(12)18-25-8-15(9-26-18)33-11-13-3-4-14(7-16(13)21)35-36(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103556

(CHEMBL3358001)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-29-28-18(35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103557

(CHEMBL3358000)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)-n2cnnn2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H18F4N10O2/c1-12-9-32(19-29-28-17(36-19)20(22,23)24)4-5-33(12)18-25-7-15(8-26-18)35-10-13-2-3-14(6-16(13)21)34-11-27-30-31-34/h2-3,6-8,11-12H,4-5,9-10H2,1H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103558

(CHEMBL3357999)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H17F4N7O2/c1-12-10-30(19-29-28-17(33-19)20(22,23)24)4-5-31(12)18-26-8-15(9-27-18)32-11-14-3-2-13(7-25)6-16(14)21/h2-3,6,8-9,12H,4-5,10-11H2,1H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103559

(CHEMBL3357998)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H23F3N6O4S/c1-14-11-29(20-28-27-18(34-20)21(22,23)24)7-8-30(14)19-25-9-17(10-26-19)33-12-15-3-5-16(6-4-15)13-35(2,31)32/h3-6,9-10,14H,7-8,11-13H2,1-2H3/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50103560

(CHEMBL3357997)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C20H21F3N6O4S/c1-13-11-28(19-27-26-17(33-19)20(21,22)23)7-8-29(13)18-24-9-15(10-25-18)32-12-14-3-5-16(6-4-14)34(2,30)31/h3-6,9-10,13H,7-8,11-12H2,1-2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 133 | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human GPR119 expressed in HEK293S cells assessed as cAMP accumulation incubated for 45 mins by HTRF assay |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data