

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 710 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088383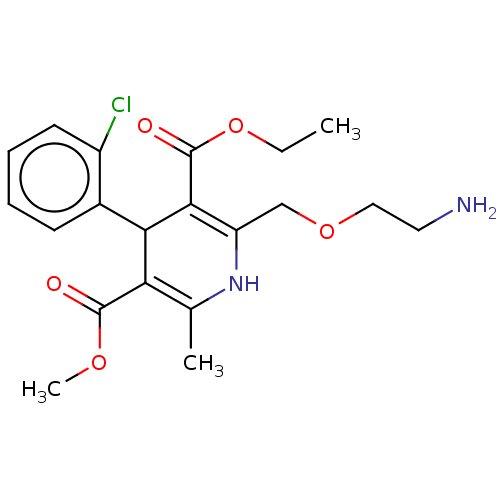 (Amlodipine | CHEBI:2668 | Norvasc) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM22876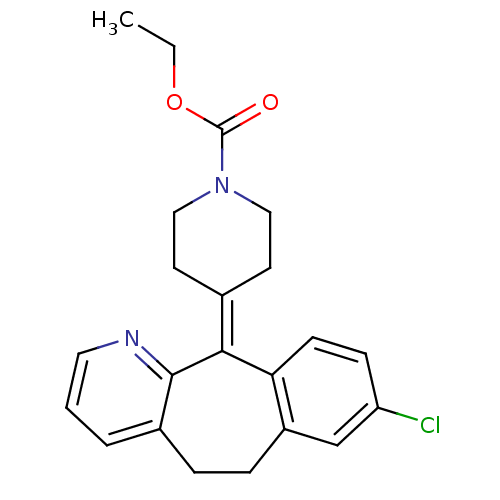 (CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50101963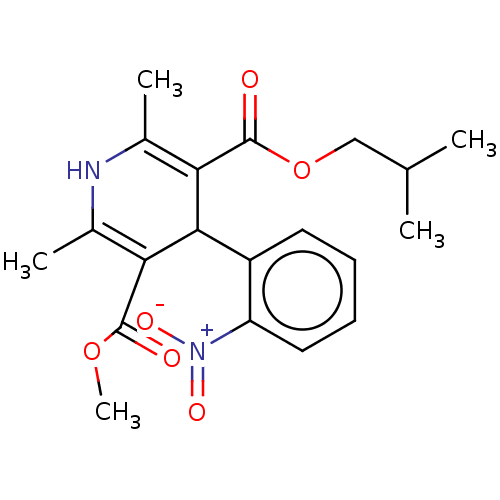 (BAY-K-5552 | CHEBI:76917 | Nisoldipine | Sular) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM115054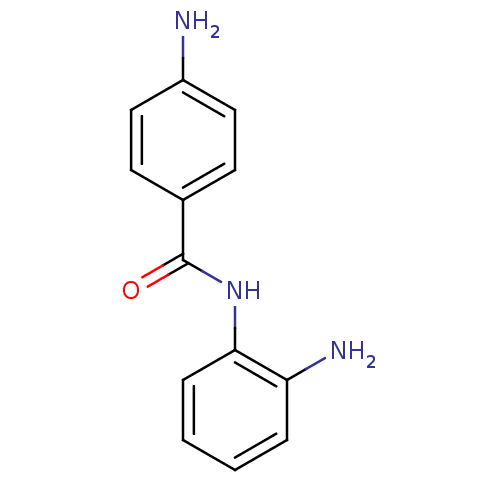 (4-Amino-N-(2-amino-phenyl)-benzamide | 4-amino-N-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM115054 (4-Amino-N-(2-amino-phenyl)-benzamide | 4-amino-N-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103608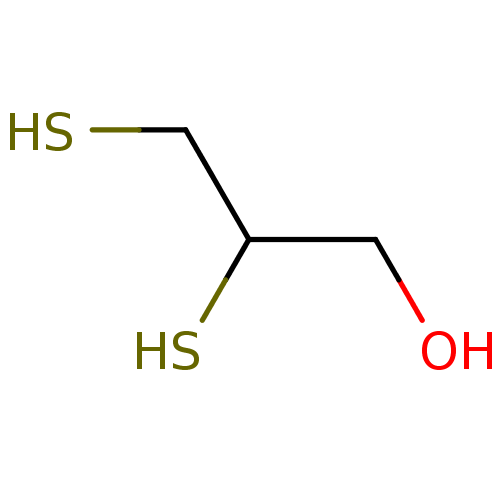 (Anti-Lewisite | CHEBI:64198 | Dimercaprol | Sulfac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103631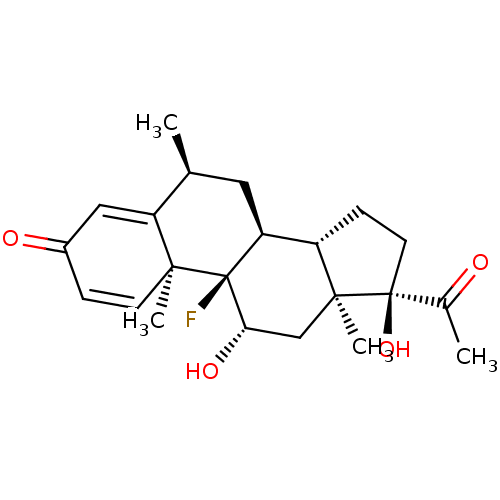 (CHEBI:31625 | Fluor-Op | Fluorometholone | Fml For...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088492 (Bumecaine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103611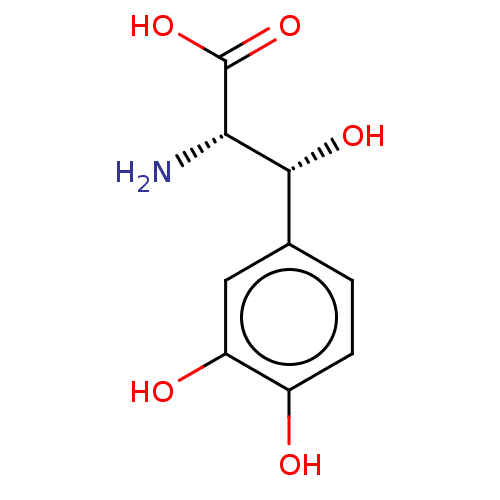 (CHEBI:31524 | DOPS | Droxidopa | L-DOPS) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103636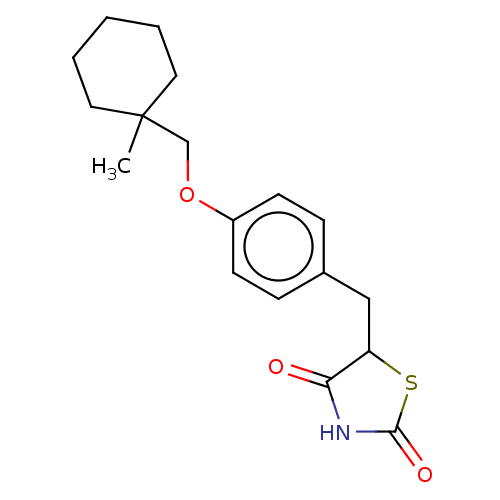 (ADD-3878 | CHEBI:64227 | Ciglitazone | U-63287) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50065387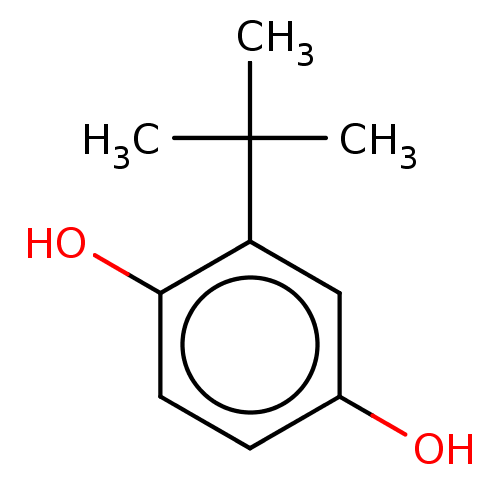 (CHEBI:78886 | E319) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103628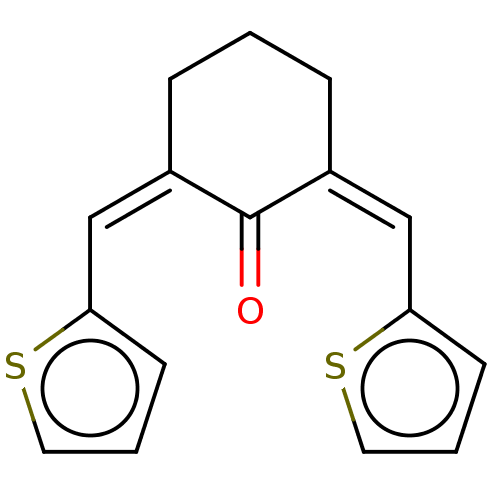 (CHEMBL1702784) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103612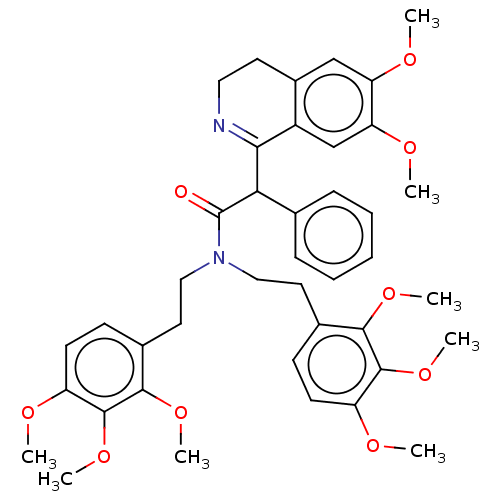 (CHEMBL2356116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50088492 (Bumecaine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50067593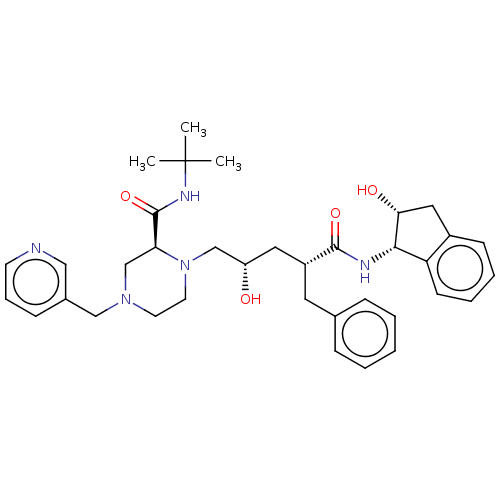 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50301375 (3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50008984 (4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM78576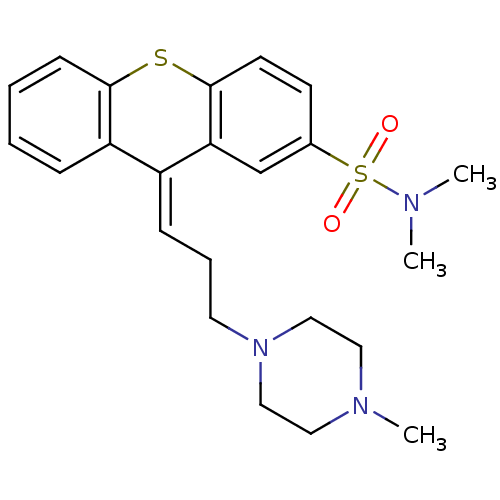 (CIS-THIOTHIXENE | MLS000028463 | SMR000058396 | TH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM32073 ((7R,11S)-11-methyl-7,15,17-tris(oxidanyl)-12-oxabi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50375318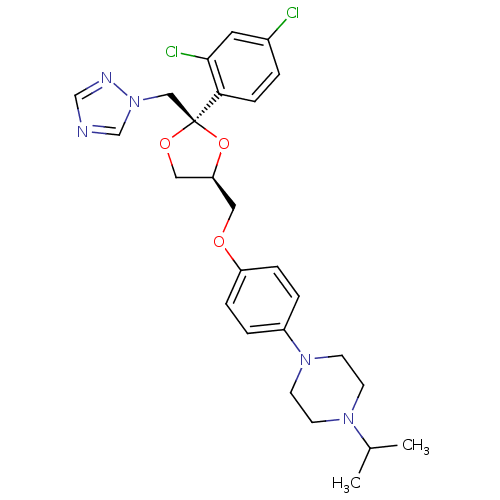 (Fungistat | Gyno-Terazol | Panlomyc | R-42470 | TE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103615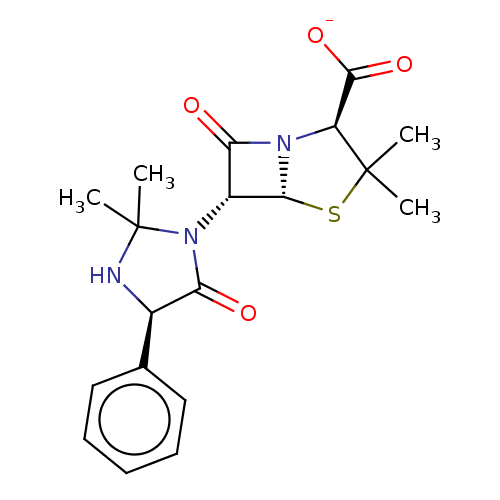 (CHEBI:34789 | Hetacillin Potassium | Versapen-K) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103614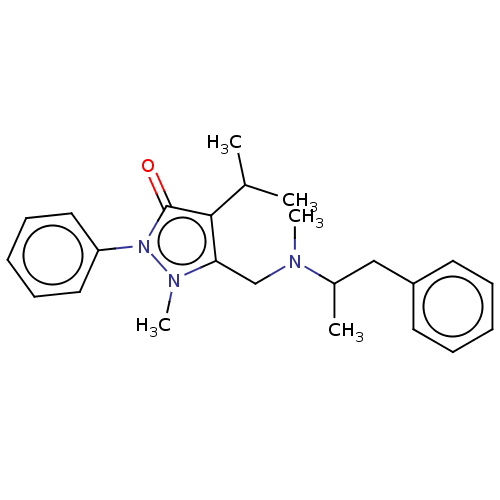 (Famprofazone) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50009073 (4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103606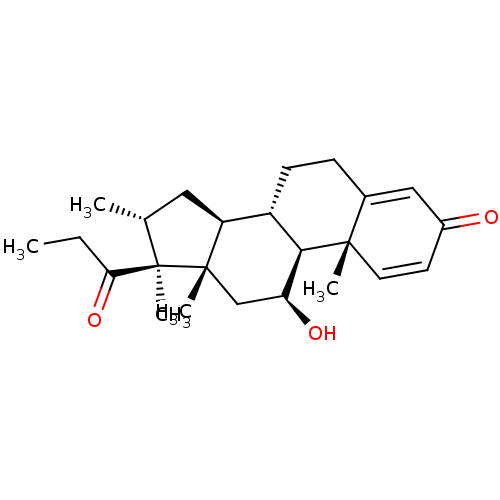 (ORG-6216 | Rimexolone | Vexol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of PXR in human cryopreserved hepatocytes assessed as induction of CYP3A4 | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103619 (CHEMBL1411951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50088493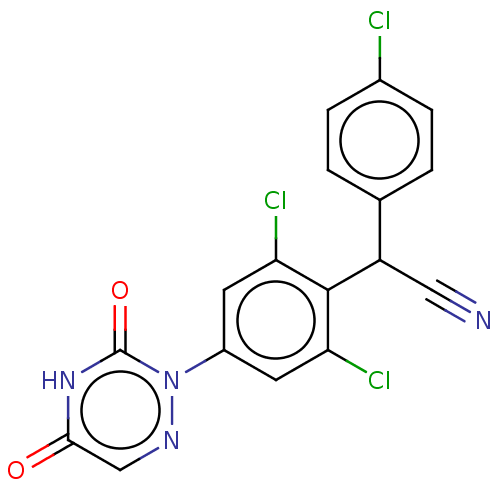 (Diclazuril | R-64433) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103612 (CHEMBL2356116) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103635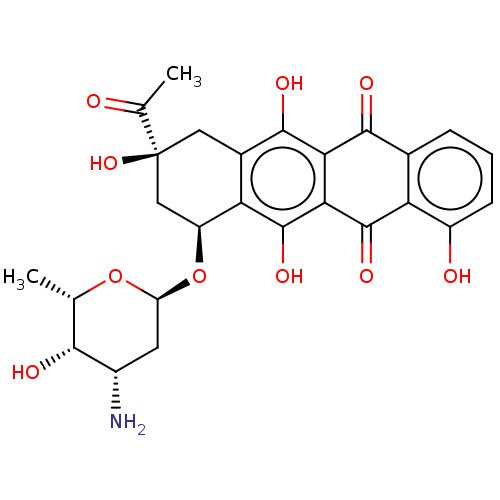 (CHEBI:31359 | Carminomycin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103633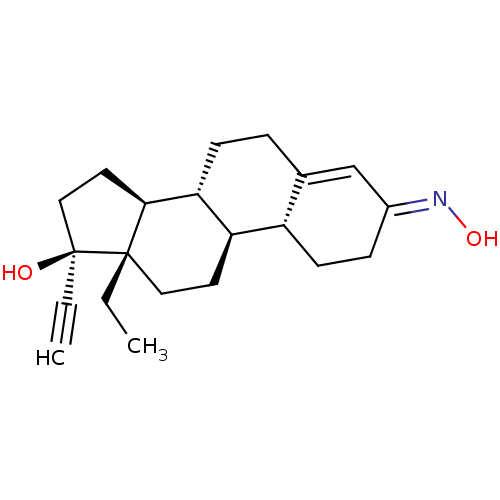 (EVRA | Norelgestromin | ORTHO EVRA | RWJ-10553) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088493 (Diclazuril | R-64433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103618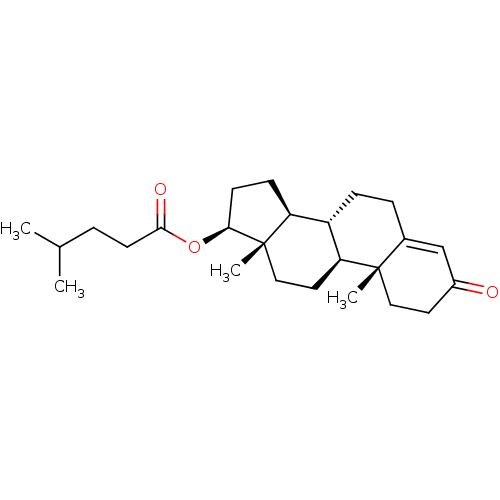 (CHEBI:35001 | CHEMBL3189011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM32073 ((7R,11S)-11-methyl-7,15,17-tris(oxidanyl)-12-oxabi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assay | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50375318 (Fungistat | Gyno-Terazol | Panlomyc | R-42470 | TE...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103634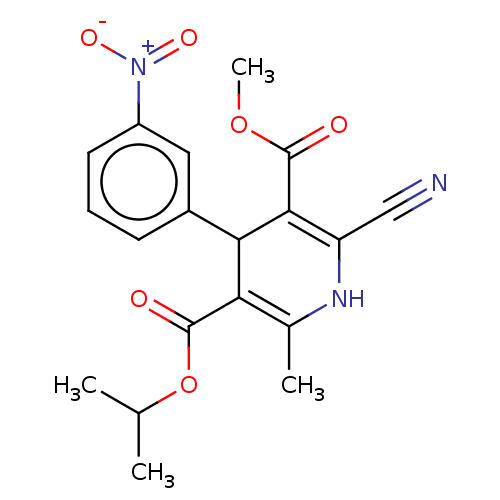 (CL-287389 | FK-235 | Nilvadipine | Nivadipine | SK...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103629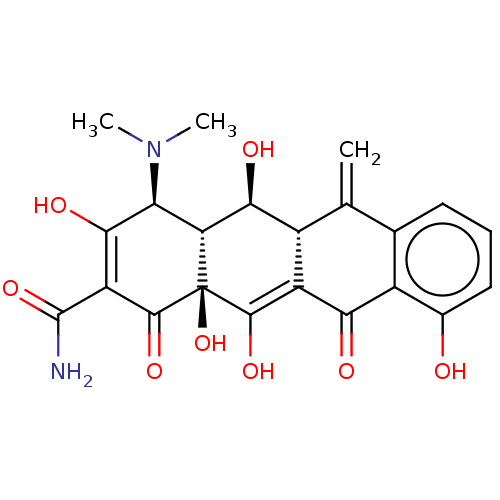 (CHEBI:6806 | METHACYCLINE HYDROCHLORIDE | Methacyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50088492 (Bumecaine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50103622 (A-41-304 | CHEBI:691037 | Desoximetasone | HOE-304...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM189379 (5-O-ethyl 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50017716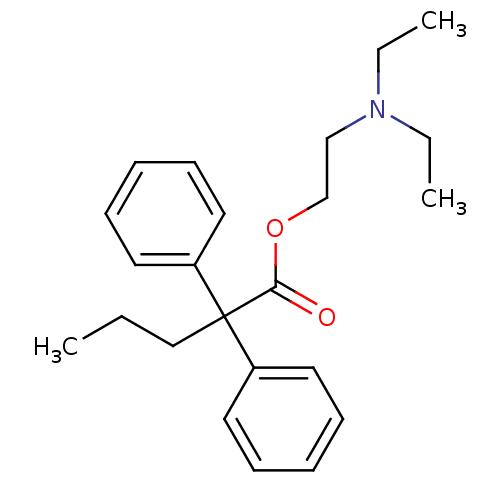 (2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM22876 (CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM32147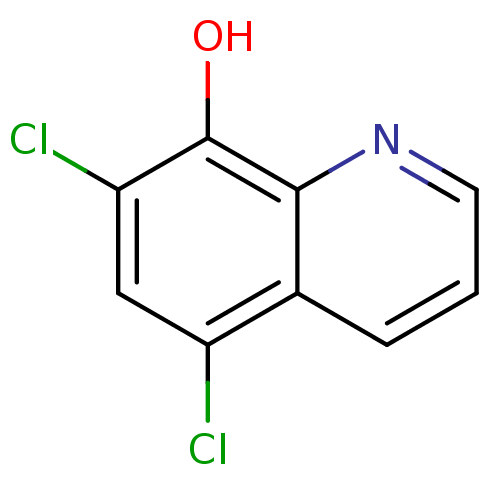 (AS-229 | CHLOROXINE | cid_2722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103631 (CHEBI:31625 | Fluor-Op | Fluorometholone | Fml For...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50103630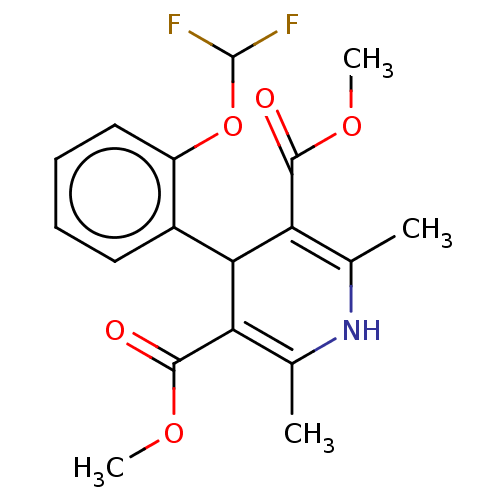 (Riodipine) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM59083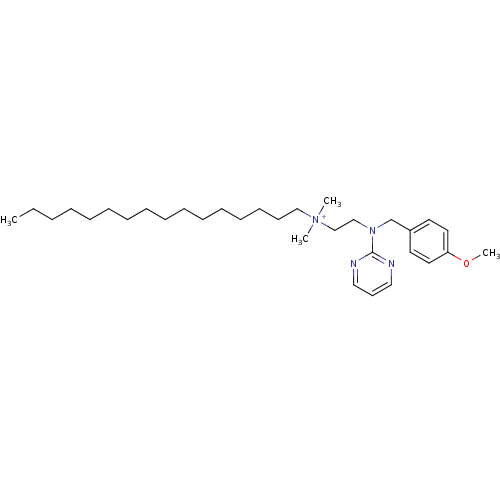 (MLS002154065 | SMR001233380 | THONZONIUM BROMIDE |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Rattus norvegicus) | BDBM50101963 (BAY-K-5552 | CHEBI:76917 | Nisoldipine | Sular) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a |
National Institutes of Health Chemical Genomics Center Curated by ChEMBL | Assay Description Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysis | Drug Metab Dispos 39: 151-9 (2010) Article DOI: 10.1124/dmd.110.035105 BindingDB Entry DOI: 10.7270/Q2JS9S5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 200 total ) | Next | Last >> |