 Found 26 hits Enz. Inhib. hit(s) with all data for entry = 50046359
Found 26 hits Enz. Inhib. hit(s) with all data for entry = 50046359 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Canis lupus familiaris) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of dog MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Mus musculus) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Canis lupus familiaris) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of dog MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Mus musculus) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mouse MDM2 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM162023
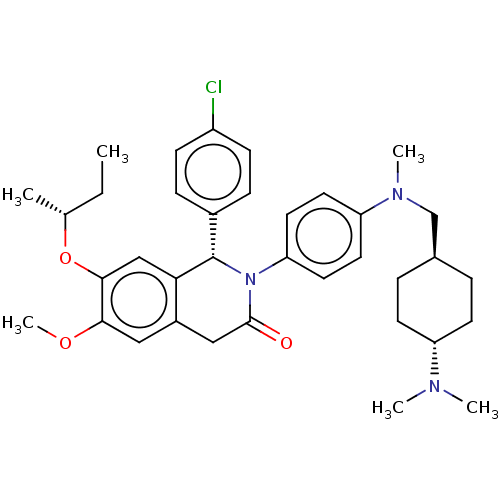
(US9051279, 73a | US9051279, 73b)Show SMILES CC[C@@H](C)Oc1cc2[C@@H](N(C(=O)Cc2cc1OC)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N(C)C)c1ccc(Cl)cc1 |r,wU:30.36,wD:8.39,2.2,27.29,(9.34,7.63,;8,6.86,;6.67,7.63,;6.67,9.17,;5.33,6.86,;4,7.63,;2.67,6.86,;1.33,7.63,;,6.86,;-1.33,7.63,;-1.33,9.17,;-2.67,9.94,;,9.94,;1.33,9.17,;2.67,9.94,;4,9.17,;5.33,9.94,;5.33,11.48,;-2.67,6.86,;-2.67,5.32,;-4,4.55,;-5.33,5.32,;-5.33,6.86,;-4,7.63,;-6.67,4.55,;-6.67,3.01,;-8,5.32,;-8,6.86,;-6.67,7.63,;-6.67,9.17,;-8,9.94,;-9.34,9.17,;-9.34,7.63,;-8,11.48,;-9.34,12.25,;-6.67,12.25,;,5.32,;-1.33,4.55,;-1.33,3.01,;,2.24,;,.7,;1.33,3.01,;1.33,4.55,)| Show InChI InChI=1S/C36H46ClN3O3/c1-7-24(2)43-34-22-32-27(20-33(34)42-6)21-35(41)40(36(32)26-10-12-28(37)13-11-26)31-18-16-30(17-19-31)39(5)23-25-8-14-29(15-9-25)38(3)4/h10-13,16-20,22,24-25,29,36H,7-9,14-15,21,23H2,1-6H3/t24-,25-,29-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by radioligand binding assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM4 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Protein Mdm4
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM4 by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50108101
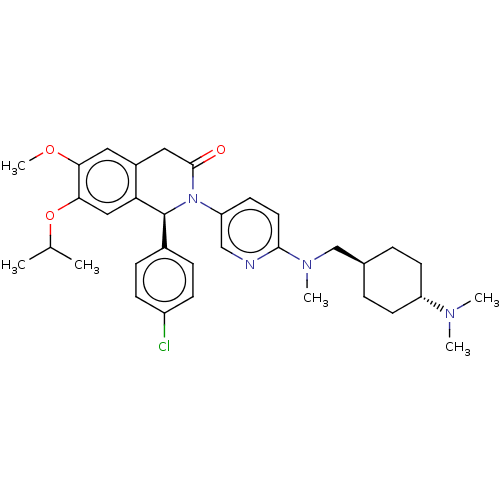
(CHEMBL3601399)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(nc1)N(C)C[C@H]1CC[C@@H](CC1)N(C)C |r,wU:36.43,wD:9.9,33.36,(-4.01,-2.77,;-4.01,-1.54,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,6.16,;1.32,7.39,;-.01,5.39,;-0,3.85,;,.77,;-1.33,1.54,;-2.68,.77,;-4.01,1.54,;-4.01,3.08,;-5.08,3.7,;-2.94,3.7,;4,1.54,;4,3.08,;5.33,3.85,;6.67,3.07,;6.66,1.53,;5.33,.77,;8,3.84,;8.01,5.07,;9.34,3.07,;10.67,3.83,;12.01,3.06,;13.34,3.83,;13.34,5.37,;12.01,6.14,;10.68,5.37,;14.68,6.14,;14.68,7.37,;15.74,5.52,)| Show InChI InChI=1S/C34H43ClN4O3/c1-22(2)42-31-19-29-25(17-30(31)41-6)18-33(40)39(34(29)24-9-11-26(35)12-10-24)28-15-16-32(36-20-28)38(5)21-23-7-13-27(14-8-23)37(3)4/h9-12,15-17,19-20,22-23,27,34H,7-8,13-14,18,21H2,1-6H3/t23-,27-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162052
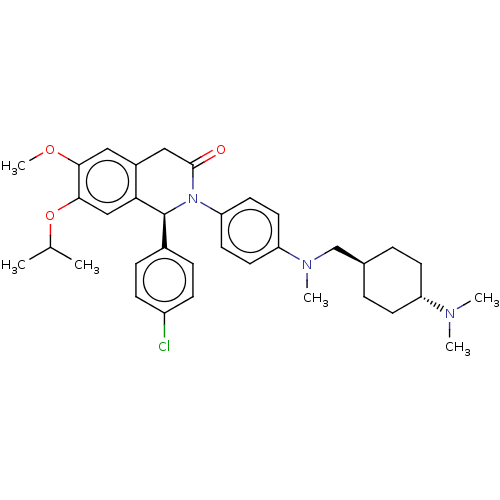
(US9051279, 75)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N(C)C |r,wU:36.43,wD:9.9,33.36,(6,5,;6,3.46,;4.67,2.69,;3.33,3.46,;2,2.69,;.67,3.46,;-.67,2.69,;-2,3.46,;-.67,1.15,;.67,.38,;.67,-1.16,;-.67,-1.93,;-.67,-3.47,;.67,-4.24,;.67,-5.77,;2,-3.47,;2,-1.93,;2,1.15,;3.33,.38,;4.67,1.15,;6,.38,;7.34,1.15,;8.67,.38,;7.34,2.69,;-2,.38,;-2,-1.16,;-3.33,-1.93,;-4.67,-1.16,;-4.67,.38,;-3.33,1.15,;-6,-1.93,;-6,-3.47,;-7.34,-1.16,;-7.34,.38,;-6,1.15,;-6,2.69,;-7.34,3.46,;-8.67,2.69,;-8.67,1.15,;-7.34,5,;-8.67,5.77,;-6,5.77,)| Show InChI InChI=1S/C35H44ClN3O3/c1-23(2)42-33-21-31-26(19-32(33)41-6)20-34(40)39(35(31)25-9-11-27(36)12-10-25)30-17-15-29(16-18-30)38(5)22-24-7-13-28(14-8-24)37(3)4/h9-12,15-19,21,23-24,28,35H,7-8,13-14,20,22H2,1-6H3/t24-,28-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162069
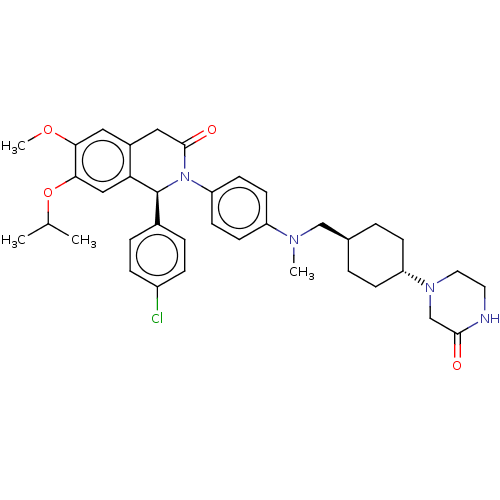
(US9051279, 79)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCNC(=O)C1 |r,wU:36.43,wD:9.9,33.36,(6,3.85,;6,2.31,;4.67,1.54,;3.33,2.31,;2,1.54,;.67,2.31,;-.67,1.54,;-2,2.31,;-.67,,;.67,-.77,;.67,-2.31,;-.67,-3.08,;-.67,-4.62,;.67,-5.39,;.67,-6.93,;2,-4.62,;2,-3.08,;2,,;3.33,-.77,;4.67,,;6,-.77,;7.34,,;8.67,-.77,;7.34,1.54,;-2,-.77,;-3.33,,;-4.67,-.77,;-4.67,-2.31,;-3.33,-3.08,;-2,-2.31,;-6,-3.08,;-6,-4.62,;-7.34,-2.31,;-7.34,-.77,;-6,,;-6,1.54,;-7.34,2.31,;-8.67,1.54,;-8.67,,;-7.34,3.85,;-8.67,4.62,;-8.67,6.16,;-7.34,6.93,;-6,6.16,;-4.67,6.93,;-6,4.62,)| Show InChI InChI=1S/C37H45ClN4O4/c1-24(2)46-34-21-32-27(19-33(34)45-4)20-36(44)42(37(32)26-7-9-28(38)10-8-26)31-15-13-29(14-16-31)40(3)22-25-5-11-30(12-6-25)41-18-17-39-35(43)23-41/h7-10,13-16,19,21,24-25,30,37H,5-6,11-12,17-18,20,22-23H2,1-4H3,(H,39,43)/t25-,30-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50108102
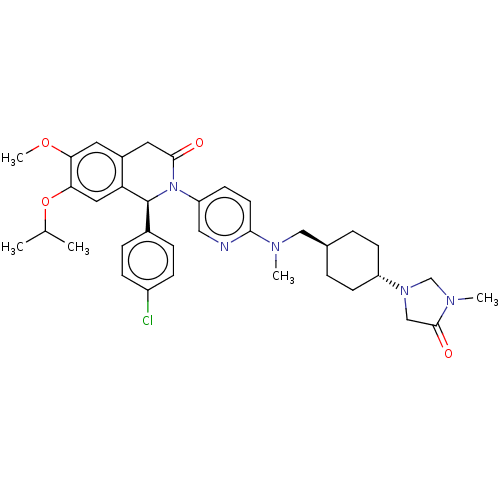
(CHEMBL3601400)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(nc1)N(C)C[C@H]1CC[C@@H](CC1)N1CN(C)C(=O)C1 |r,wU:36.43,wD:9.9,33.36,(-4.01,-2.77,;-4.01,-1.54,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,6.16,;1.32,7.39,;-.01,5.39,;-0,3.85,;,.77,;-1.33,1.54,;-2.68,.77,;-4.01,1.54,;-4.01,3.08,;-5.08,3.7,;-2.94,3.7,;4,1.54,;4,3.08,;5.33,3.85,;6.67,3.07,;6.66,1.53,;5.33,.77,;8,3.84,;8.01,5.07,;9.34,3.07,;10.67,3.83,;12.01,3.06,;13.34,3.83,;13.34,5.37,;12.01,6.14,;10.68,5.37,;14.68,6.14,;14.82,7.66,;16.33,7.97,;16.84,9.09,;17.1,6.63,;18.32,6.5,;16.06,5.49,)| Show InChI InChI=1S/C36H44ClN5O4/c1-23(2)46-32-18-30-26(16-31(32)45-5)17-34(43)42(36(30)25-8-10-27(37)11-9-25)29-14-15-33(38-19-29)39(3)20-24-6-12-28(13-7-24)41-21-35(44)40(4)22-41/h8-11,14-16,18-19,23-24,28,36H,6-7,12-13,17,20-22H2,1-5H3/t24-,28-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM162069

(US9051279, 79)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCNC(=O)C1 |r,wU:36.43,wD:9.9,33.36,(6,3.85,;6,2.31,;4.67,1.54,;3.33,2.31,;2,1.54,;.67,2.31,;-.67,1.54,;-2,2.31,;-.67,,;.67,-.77,;.67,-2.31,;-.67,-3.08,;-.67,-4.62,;.67,-5.39,;.67,-6.93,;2,-4.62,;2,-3.08,;2,,;3.33,-.77,;4.67,,;6,-.77,;7.34,,;8.67,-.77,;7.34,1.54,;-2,-.77,;-3.33,,;-4.67,-.77,;-4.67,-2.31,;-3.33,-3.08,;-2,-2.31,;-6,-3.08,;-6,-4.62,;-7.34,-2.31,;-7.34,-.77,;-6,,;-6,1.54,;-7.34,2.31,;-8.67,1.54,;-8.67,,;-7.34,3.85,;-8.67,4.62,;-8.67,6.16,;-7.34,6.93,;-6,6.16,;-4.67,6.93,;-6,4.62,)| Show InChI InChI=1S/C37H45ClN4O4/c1-24(2)46-34-21-32-27(19-33(34)45-4)20-36(44)42(37(32)26-7-9-28(38)10-8-26)31-15-13-29(14-16-31)40(3)22-25-5-11-30(12-6-25)41-18-17-39-35(43)23-41/h7-10,13-16,19,21,24-25,30,37H,5-6,11-12,17-18,20,22-23H2,1-4H3,(H,39,43)/t25-,30-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by radioligand binding assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162157
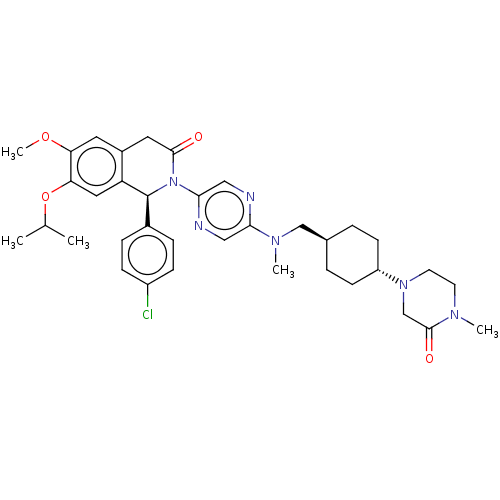
(US9051279, 140)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1cnc(cn1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:36.43,wD:9.9,33.36,(12,4.23,;10.67,5,;9.34,4.23,;8,5,;6.67,4.23,;5.33,5,;4,4.23,;2.67,5,;4,2.69,;5.33,1.93,;5.33,.38,;4,-.38,;4,-1.93,;5.33,-2.69,;5.33,-4.23,;6.67,-1.93,;6.67,-.38,;6.67,2.69,;8,1.93,;9.34,2.69,;10.67,1.93,;10.67,.38,;12,-.38,;9.34,-.38,;2.67,1.93,;2.67,.38,;1.33,-.38,;,.38,;,1.93,;1.33,2.69,;-1.33,-.38,;-1.33,-1.93,;-2.67,.38,;-4,-.38,;-5.33,.38,;-6.67,-.38,;-6.67,-1.93,;-5.33,-2.69,;-4,-1.93,;-8,-2.69,;-8,-4.23,;-9.34,-5,;-10.67,-4.23,;-12,-5,;-10.67,-2.69,;-12,-1.93,;-9.34,-1.93,)| Show InChI InChI=1S/C36H45ClN6O4/c1-23(2)47-31-18-29-26(16-30(31)46-5)17-34(44)43(36(29)25-8-10-27(37)11-9-25)33-20-38-32(19-39-33)41(4)21-24-6-12-28(13-7-24)42-15-14-40(3)35(45)22-42/h8-11,16,18-20,23-24,28,36H,6-7,12-15,17,21-22H2,1-5H3/t24-,28-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162142
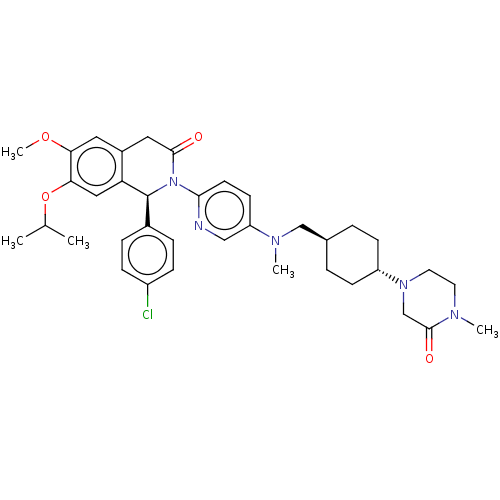
(US9051279, 125)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cn1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:36.43,wD:9.9,33.36,(6,3.08,;6,1.54,;4.67,.77,;3.33,1.54,;2,.77,;.67,1.54,;-.67,.77,;-2,1.54,;-.67,-.77,;.67,-1.54,;.67,-3.08,;-.67,-3.85,;-.67,-5.39,;.67,-6.16,;.67,-7.7,;2,-5.39,;2,-3.85,;2,-.77,;3.33,-1.54,;4.67,-.77,;6,-1.54,;7.34,-.77,;8.67,-1.54,;7.34,.77,;-2,-1.54,;-2,-3.08,;-3.33,-3.85,;-4.67,-3.08,;-4.67,-1.54,;-3.33,-.77,;-6,-3.85,;-6,-5.39,;-7.34,-3.08,;-7.34,-1.54,;-6,-.77,;-6,.77,;-7.34,1.54,;-8.67,.77,;-8.67,-.77,;-7.34,3.08,;-8.67,3.85,;-8.67,5.39,;-7.34,6.16,;-7.34,7.7,;-6,5.39,;-4.67,6.16,;-6,3.85,)| Show InChI InChI=1S/C37H46ClN5O4/c1-24(2)47-33-20-31-27(18-32(33)46-5)19-35(44)43(37(31)26-8-10-28(38)11-9-26)34-15-14-30(21-39-34)41(4)22-25-6-12-29(13-7-25)42-17-16-40(3)36(45)23-42/h8-11,14-15,18,20-21,24-25,29,37H,6-7,12-13,16-17,19,22-23H2,1-5H3/t25-,29-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM162147

(US9051279, 130)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(nc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:36.43,wD:9.9,33.36,(12,5,;10.67,5.78,;9.34,5,;8,5.78,;6.67,5,;5.33,5.78,;4,5,;2.67,5.78,;4,3.47,;5.33,2.69,;5.33,1.15,;4,.38,;4,-1.15,;5.33,-1.93,;5.33,-3.47,;6.67,-1.15,;6.67,.38,;6.67,3.47,;8,2.69,;9.34,3.47,;10.67,2.69,;10.67,1.15,;12,.38,;9.34,.38,;2.67,2.69,;2.67,1.15,;1.33,.38,;,1.15,;,2.69,;1.33,3.47,;-1.33,.38,;-1.33,-1.15,;-2.67,1.15,;-4,.38,;-5.33,1.15,;-6.67,.38,;-6.67,-1.15,;-5.33,-1.93,;-4,-1.15,;-8,-1.93,;-9.34,-1.15,;-10.67,-1.93,;-10.67,-3.47,;-12,-4.23,;-9.34,-4.23,;-9.34,-5.78,;-8,-3.47,)| Show InChI InChI=1S/C37H46ClN5O4/c1-24(2)47-33-20-31-27(18-32(33)46-5)19-35(44)43(37(31)26-8-10-28(38)11-9-26)30-14-15-34(39-21-30)41(4)22-25-6-12-29(13-7-25)42-17-16-40(3)36(45)23-42/h8-11,14-15,18,20-21,24-25,29,37H,6-7,12-13,16-17,19,22-23H2,1-5H3/t25-,29-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50108100
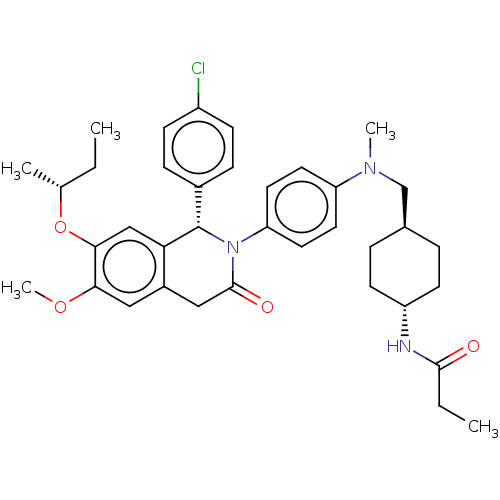
(CHEMBL3601324)Show SMILES CC[C@@H](C)Oc1cc2[C@@H](N(C(=O)Cc2cc1OC)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)NC(=O)CC)c1ccc(Cl)cc1 |r,wU:2.2,30.36,wD:8.41,27.29,(-5.34,5.09,;-5.34,3.86,;-4.01,3.08,;-2.94,3.7,;-4.01,1.54,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-4.01,-1.54,;-4.01,-2.77,;4,1.54,;4,3.08,;5.33,3.85,;6.67,3.07,;6.66,1.53,;5.33,.77,;8,3.84,;8.01,5.07,;9.34,3.07,;10.67,3.83,;12.01,3.06,;13.34,3.83,;13.34,5.37,;12.01,6.14,;10.68,5.37,;14.68,6.13,;16.01,5.36,;16.01,4.13,;17.35,6.12,;18.41,5.5,;1.33,3.08,;2.66,3.85,;2.66,5.39,;1.33,6.16,;1.32,7.39,;-.01,5.39,;-0,3.85,)| Show InChI InChI=1S/C37H46ClN3O4/c1-6-24(3)45-34-22-32-27(20-33(34)44-5)21-36(43)41(37(32)26-10-12-28(38)13-11-26)31-18-16-30(17-19-31)40(4)23-25-8-14-29(15-9-25)39-35(42)7-2/h10-13,16-20,22,24-25,29,37H,6-9,14-15,21,23H2,1-5H3,(H,39,42)/t24-,25-,29-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by radioligand binding assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) assessed as inhibition of Bcl2-Bak interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) assessed as inhibition of Bcl2-Bad interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) assessed as inhibition of Mcl1-Bak interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) assessed as inhibition of Mcl1-Noxa interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of XIAP (unknown origin) assessed as inhibition of XIAP-BIR3 interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cIAP (unknown origin) assessed as inhibition of cIAP-BIR3 interaction by TR-FRET assay |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM162123

(US9051279, 106)Show SMILES COc1cc2CC(=O)N([C@@H](c3ccc(Cl)cc3)c2cc1OC(C)C)c1ccc(cc1)N(C)C[C@H]1CC[C@@H](CC1)N1CCN(C)C(=O)C1 |r,wU:33.36,wD:9.9,36.43,(8.67,.77,;7.34,1.54,;6,.77,;4.67,1.54,;3.33,.77,;2,1.54,;.67,.77,;-.67,1.54,;.67,-.77,;2,-1.54,;2,-3.08,;.67,-3.85,;.67,-5.39,;2,-6.16,;2,-7.7,;3.33,-5.39,;3.33,-3.85,;3.33,-.77,;4.67,-1.54,;6,-.77,;7.34,-1.54,;7.34,-3.08,;8.67,-3.85,;6,-3.85,;-.67,-1.54,;-2,-.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.67,-3.08,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-1.54,;-7.34,-.77,;-7.34,.77,;-6,1.54,;-4.67,.77,;-4.67,-.77,;-6,3.08,;-4.67,3.85,;-4.67,5.39,;-6,6.16,;-6,7.7,;-7.34,5.39,;-8.67,6.16,;-7.34,3.85,)| Show InChI InChI=1S/C38H47ClN4O4/c1-25(2)47-35-22-33-28(20-34(35)46-5)21-36(44)43(38(33)27-8-10-29(39)11-9-27)32-16-14-30(15-17-32)41(4)23-26-6-12-31(13-7-26)42-19-18-40(3)37(45)24-42/h8-11,14-17,20,22,25-26,31,38H,6-7,12-13,18-19,21,23-24H2,1-5H3/t26-,31-,38-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MDM2 L33E mutant (14 to 111 residues) by ITC method |
J Med Chem 58: 6348-58 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00810
BindingDB Entry DOI: 10.7270/Q2HQ41QV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data