

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM50215709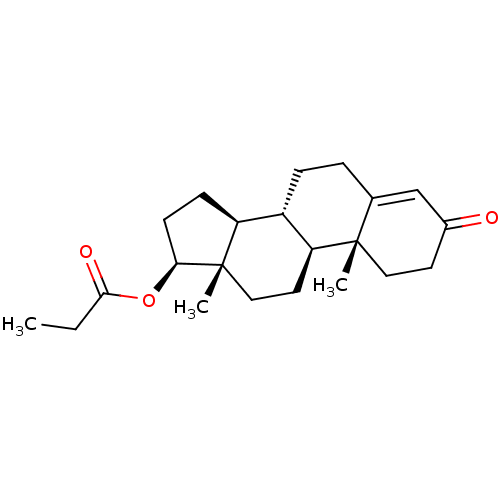 (CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215713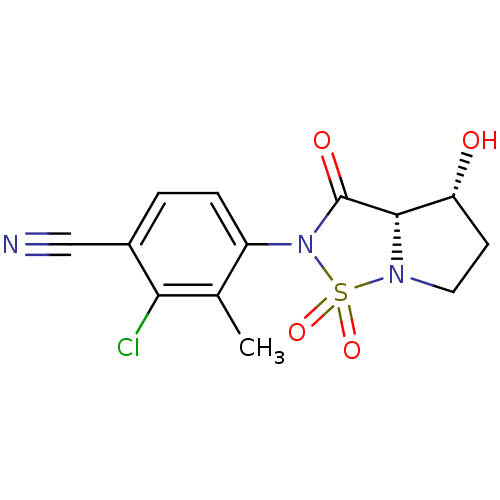 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18173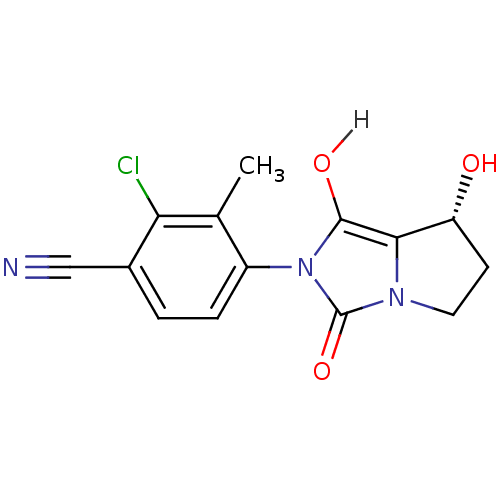 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215716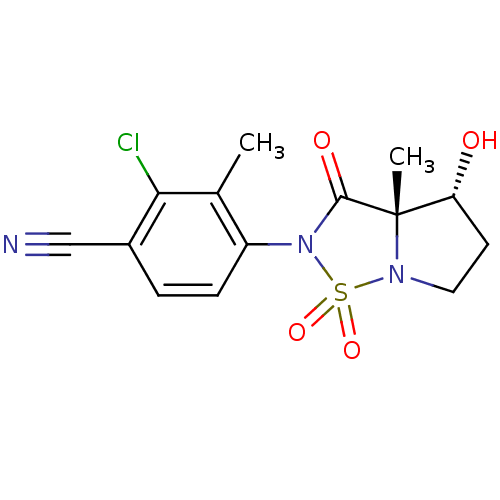 (2-chloro-4-((3aS,4R)-4-hydroxy-3a-methyl-1,1,3-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215714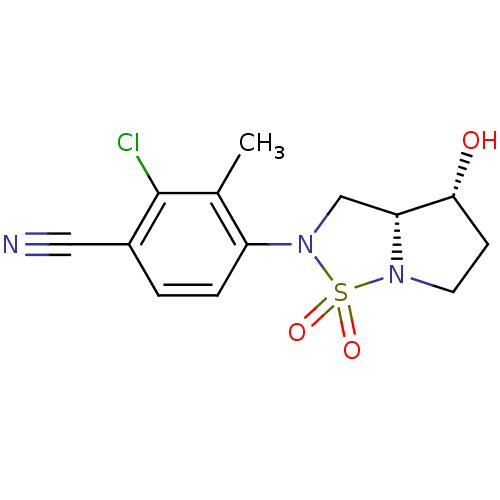 (2-chloro-4-((3aR,4R)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215710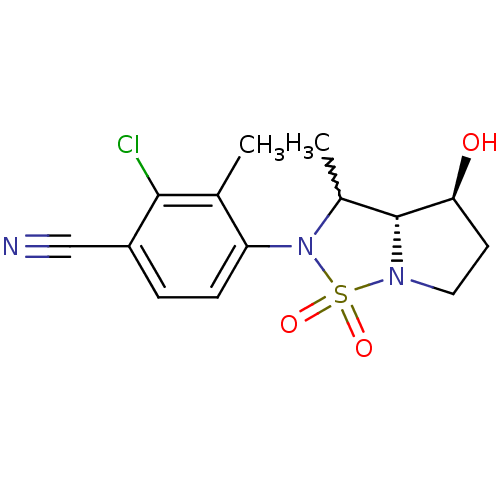 (2-chloro-4-((3aR,4S)-4-hydroxy-3-methyl-1,1-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215712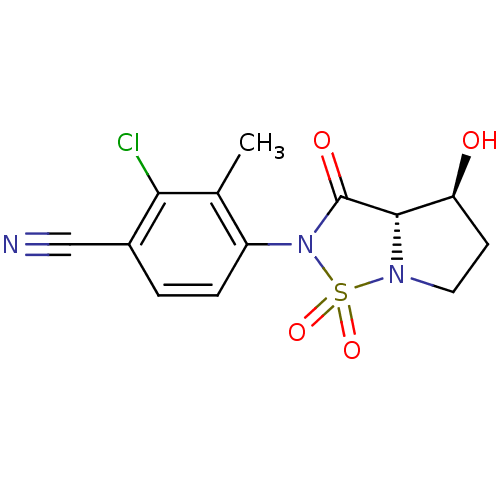 (2-chloro-4-((3aS,4S)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215718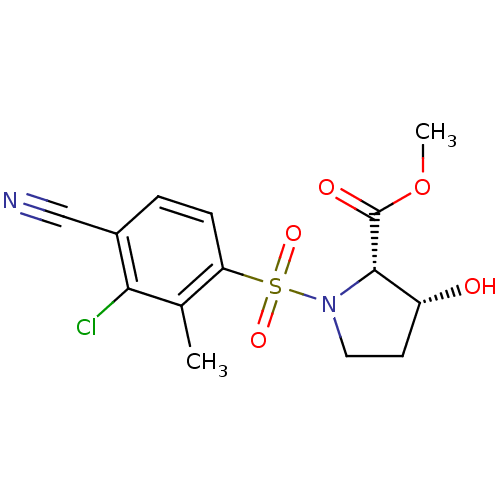 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215711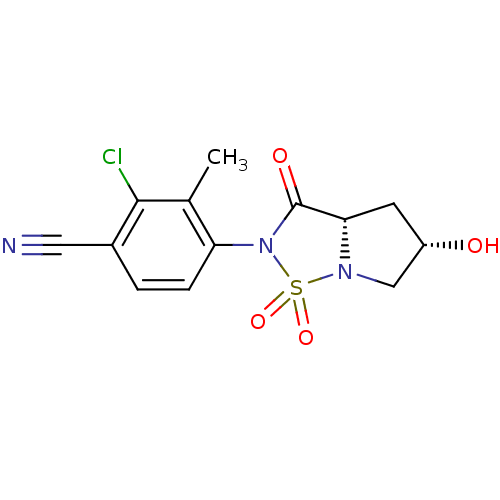 (2-chloro-4-((3aS,5S)-5-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50215719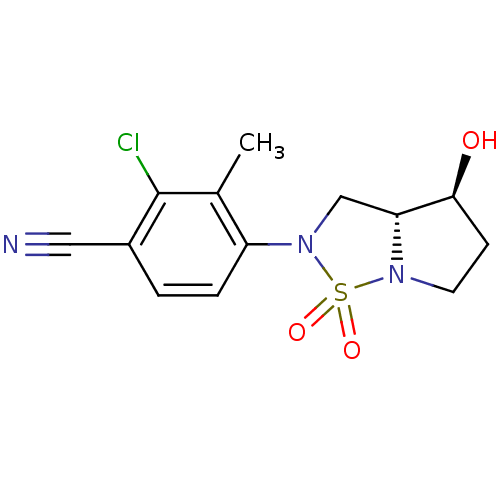 (2-chloro-4-((3aR,4S)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from human androgen receptor in MDA453 cells | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215718 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215713 (2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11.9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215715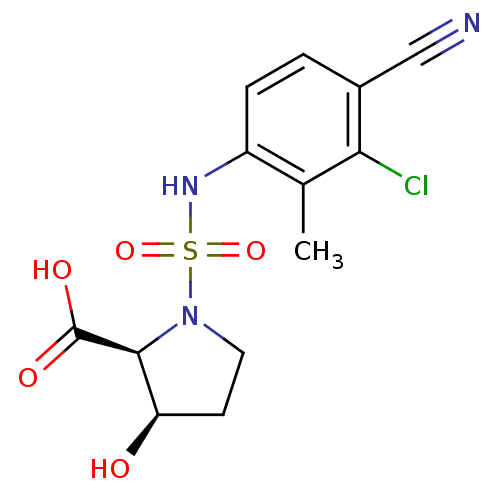 ((2S,3R)-1-(3-chloro-4-cyano-2-methyl-phenylsulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215709 (CHEMBL1170 | Propionic acid (8R,9S,10R,13S,14S,17S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215716 (2-chloro-4-((3aS,4R)-4-hydroxy-3a-methyl-1,1,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215717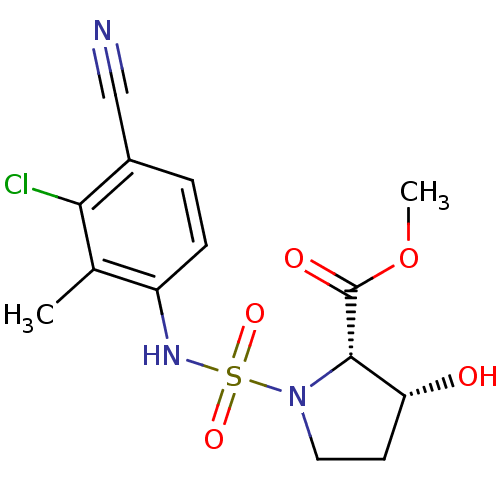 ((2S,3R)-1-(3-chloro-4-cyano-2-methyl-phenylsulfamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.46E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215718 ((2S,3R)-methyl 1-(3-chloro-4-cyano-2-methylphenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215714 (2-chloro-4-((3aR,4R)-4-hydroxy-1,1-dioxo-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.73E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215712 (2-chloro-4-((3aS,4S)-4-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM18173 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215711 (2-chloro-4-((3aS,5S)-5-hydroxy-1,1,3-trioxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Mus musculus) | BDBM50215710 (2-chloro-4-((3aR,4S)-4-hydroxy-3-methyl-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.96E+3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in mouse C2C12 cells by receptor transactivation assay | Bioorg Med Chem Lett 17: 4487-90 (2007) Article DOI: 10.1016/j.bmcl.2007.06.007 BindingDB Entry DOI: 10.7270/Q2NV9HZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||