

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110732 (CHEMBL3605927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.998 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110730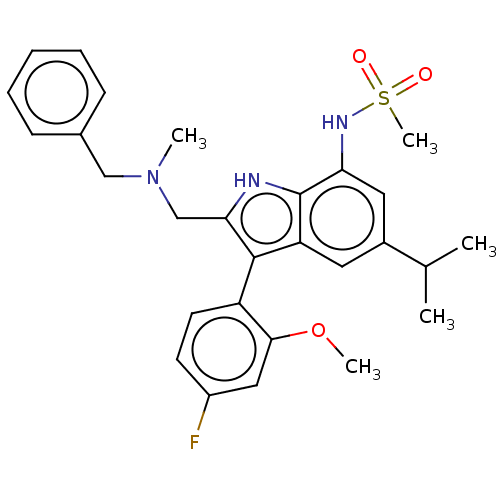 (CHEMBL3605919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110733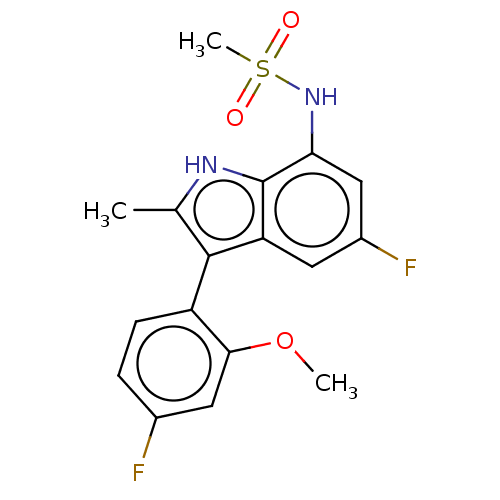 (CHEMBL3605929) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110735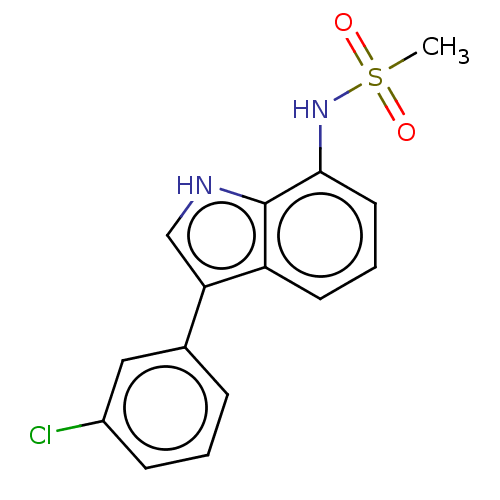 (CHEMBL3605932) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110731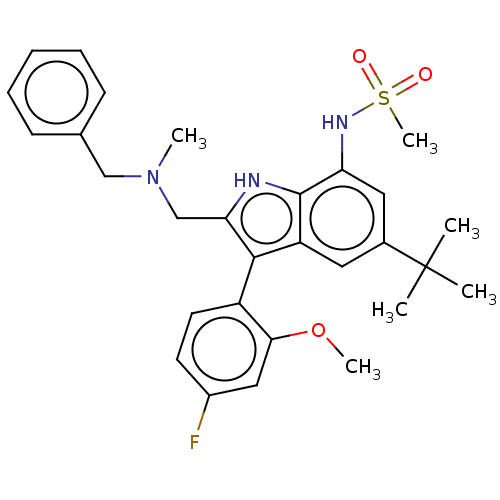 (CHEMBL3605926) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110736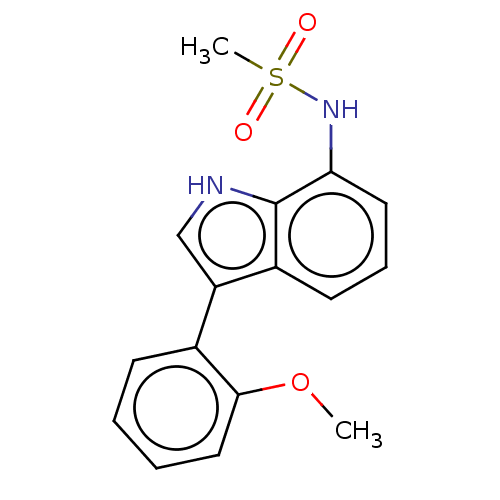 (CHEMBL3605933) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110735 (CHEMBL3605932) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110734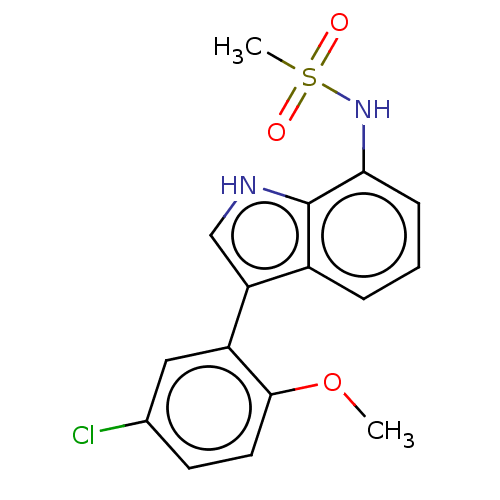 (CHEMBL3605930) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110735 (CHEMBL3605932) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110733 (CHEMBL3605929) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110743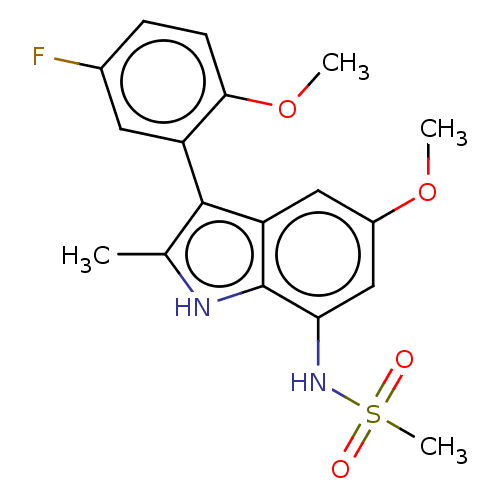 (CHEMBL3605924) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110736 (CHEMBL3605933) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110789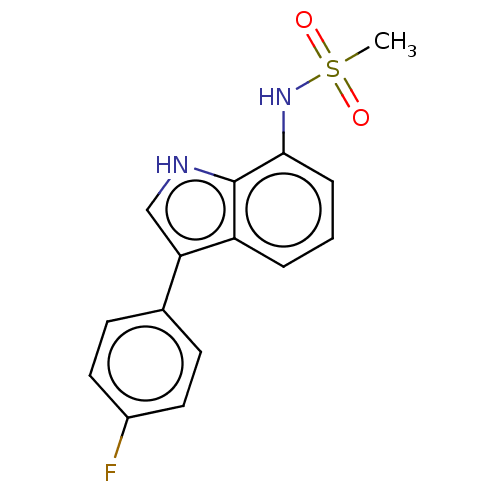 (CHEMBL3605934) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110743 (CHEMBL3605924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110739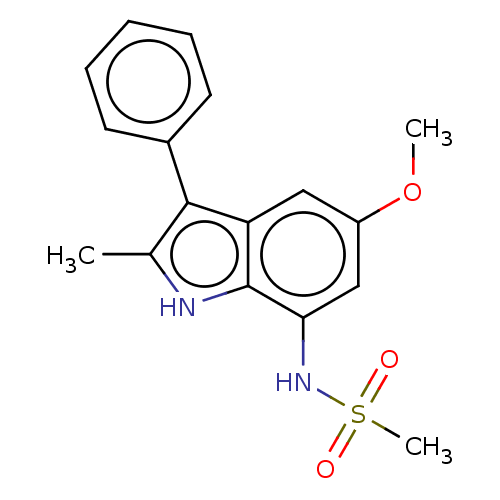 (CHEMBL3605923) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110751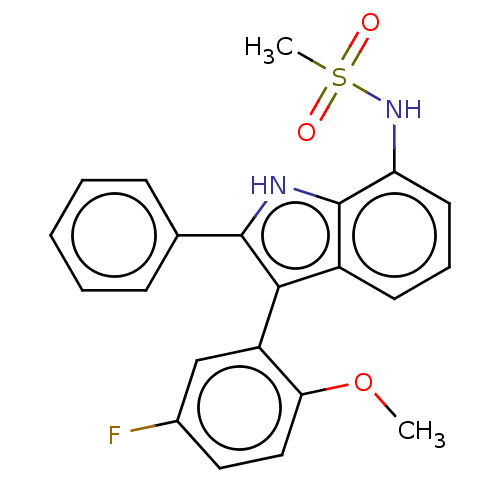 (CHEMBL3605928) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110733 (CHEMBL3605929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110750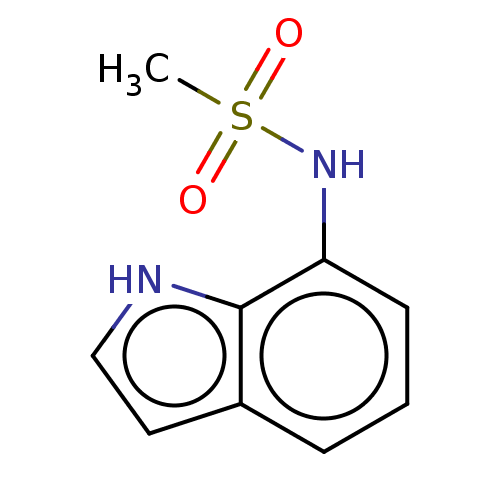 (CHEMBL3605925) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110751 (CHEMBL3605928) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110736 (CHEMBL3605933) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110735 (CHEMBL3605932) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110789 (CHEMBL3605934) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110750 (CHEMBL3605925) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110743 (CHEMBL3605924) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110789 (CHEMBL3605934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110731 (CHEMBL3605926) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110734 (CHEMBL3605930) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 233 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110731 (CHEMBL3605926) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110750 (CHEMBL3605925) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110736 (CHEMBL3605933) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110789 (CHEMBL3605934) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 496 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110738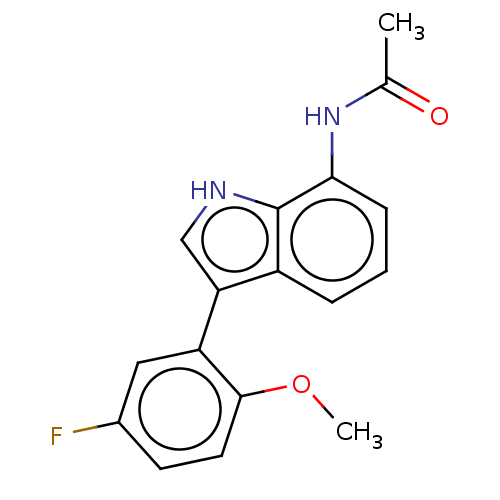 (CHEMBL3605922) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110732 (CHEMBL3605927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 684 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110738 (CHEMBL3605922) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-progesterone from human progesterone receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110734 (CHEMBL3605930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 697 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110751 (CHEMBL3605928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 905 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110750 (CHEMBL3605925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 952 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110743 (CHEMBL3605924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50110733 (CHEMBL3605929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells by scintillation counting based radioligand competition ... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50110790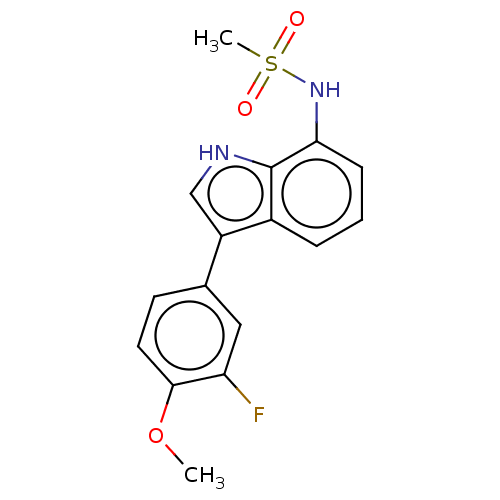 (CHEMBL3605931) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competiti... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110730 (CHEMBL3605919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110737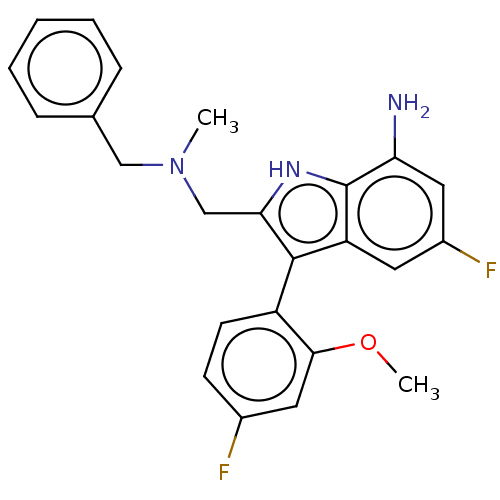 (CHEMBL3605921) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110731 (CHEMBL3605926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50110739 (CHEMBL3605923) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly Biotechnology Center Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells by scintillation counting based radioligand competit... | J Med Chem 58: 6607-18 (2015) Article DOI: 10.1021/acs.jmedchem.5b00736 BindingDB Entry DOI: 10.7270/Q2ST7RMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |