 Found 92 hits Enz. Inhib. hit(s) with all data for entry = 50020856
Found 92 hits Enz. Inhib. hit(s) with all data for entry = 50020856 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218305

(3-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1[C@@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)34-28-22-6-2-1-5-19(22)15-26(28)33-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,37)(H,34,38)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218298
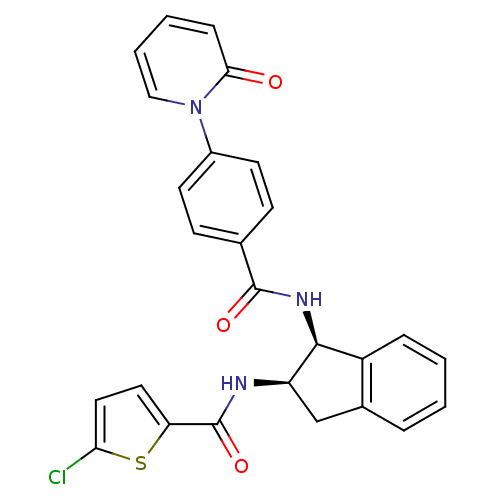
(5-chloro-N-((1S,2R)-1-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1Cc2ccccc2[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H20ClN3O3S/c27-22-13-12-21(34-22)26(33)28-20-15-17-5-1-2-6-19(17)24(20)29-25(32)16-8-10-18(11-9-16)30-14-4-3-7-23(30)31/h1-14,20,24H,15H2,(H,28,33)(H,29,32)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218280

(5-chloro-N-((3R,4S)-1-(2-methoxyacetyl)-4-(4-(2-ox...)Show SMILES COCC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-34-14-22(31)28-12-17(18(13-28)27-24(33)19-9-10-20(25)35-19)26-23(32)15-5-7-16(8-6-15)29-11-3-2-4-21(29)30/h2-11,17-18H,12-14H2,1H3,(H,26,32)(H,27,33)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218315

((3R,4S)-methyl 3-(2-chlorothiophene-5-carboxamido)...)Show SMILES COC(=O)C1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O |w:4.3| Show InChI InChI=1S/C24H22ClN3O5S/c1-33-24(32)15-12-17(18(13-15)27-23(31)19-9-10-20(25)34-19)26-22(30)14-5-7-16(8-6-14)28-11-3-2-4-21(28)29/h2-11,15,17-18H,12-13H2,1H3,(H,26,30)(H,27,31)/t15?,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216554
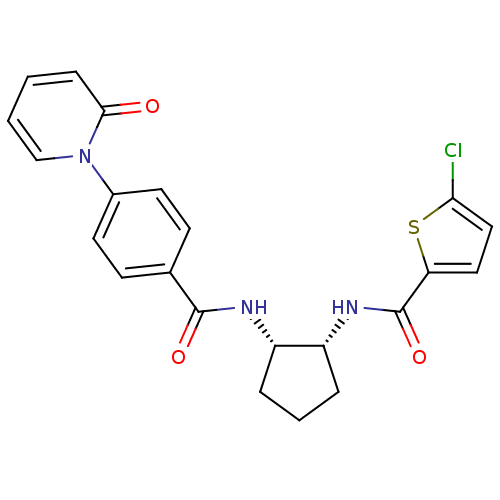
(5-CHLORO-N-((1R,2S)-2-(4-(2-OXOPYRIDIN-1(2H)-YL)BE...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H20ClN3O3S/c23-19-12-11-18(30-19)22(29)25-17-5-3-4-16(17)24-21(28)14-7-9-15(10-8-14)26-13-2-1-6-20(26)27/h1-2,6-13,16-17H,3-5H2,(H,24,28)(H,25,29)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218308
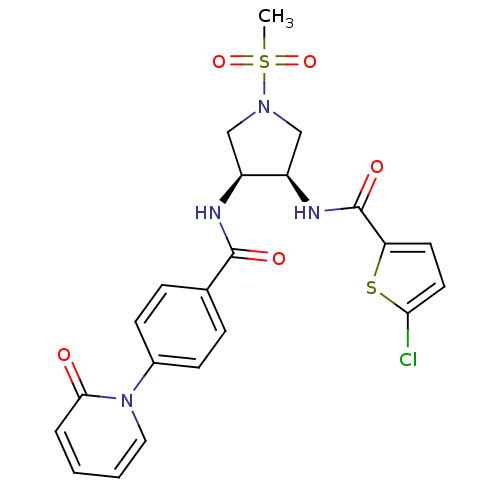
(5-chloro-N-((3R,4S)-1-(methylsulfonyl)-4-(4-(2-oxo...)Show SMILES CS(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C22H21ClN4O5S2/c1-34(31,32)26-12-16(17(13-26)25-22(30)18-9-10-19(23)33-18)24-21(29)14-5-7-15(8-6-14)27-11-3-2-4-20(27)28/h2-11,16-17H,12-13H2,1H3,(H,24,29)(H,25,30)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282
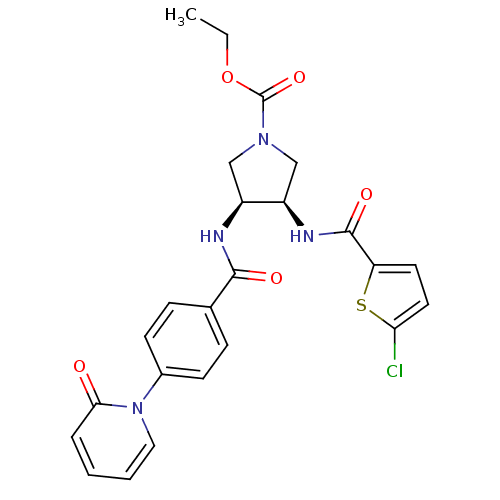
((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218312
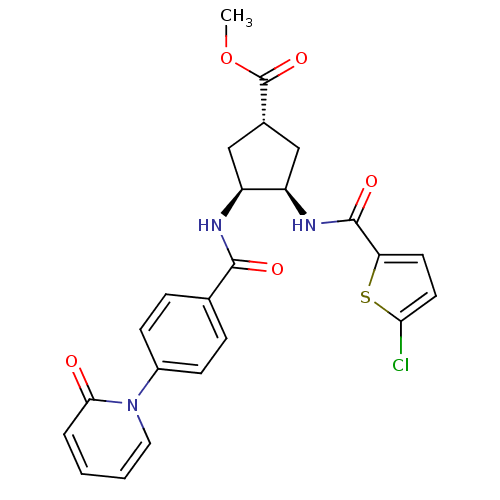
((1S,3R,4S)-methyl 3-(2-chlorothiophene-5-carboxami...)Show SMILES COC(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H22ClN3O5S/c1-33-24(32)15-12-17(18(13-15)27-23(31)19-9-10-20(25)34-19)26-22(30)14-5-7-16(8-6-14)28-11-3-2-4-21(28)29/h2-11,15,17-18H,12-13H2,1H3,(H,26,30)(H,27,31)/t15-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218300
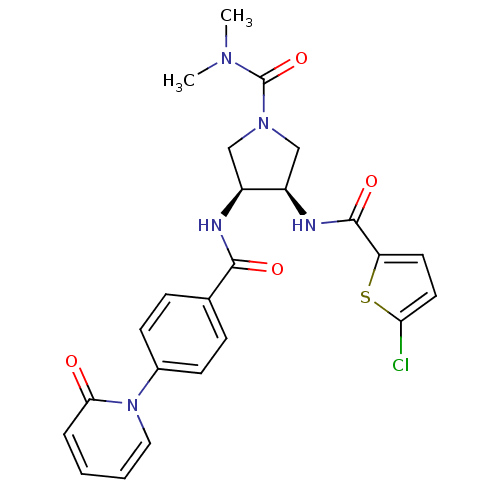
((3R,4S)-3-(2-chlorothiophene-5-carboxamido)-N,N-di...)Show SMILES CN(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H24ClN5O4S/c1-28(2)24(34)29-13-17(18(14-29)27-23(33)19-10-11-20(25)35-19)26-22(32)15-6-8-16(9-7-15)30-12-4-3-5-21(30)31/h3-12,17-18H,13-14H2,1-2H3,(H,26,32)(H,27,33)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218290
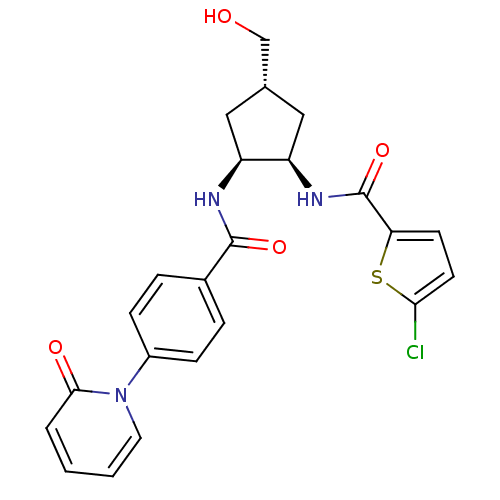
(5-chloro-N-((1R,2S,4S)-4-(hydroxymethyl)-2-(4-(2-o...)Show SMILES OC[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN3O4S/c24-20-9-8-19(32-20)23(31)26-18-12-14(13-28)11-17(18)25-22(30)15-4-6-16(7-5-15)27-10-2-1-3-21(27)29/h1-10,14,17-18,28H,11-13H2,(H,25,30)(H,26,31)/t14-,17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218291
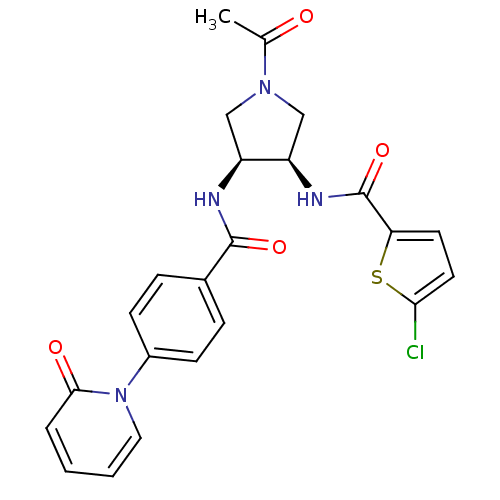
(CHEMBL394129 | N-((3R,4S)-1-acetyl-4-(4-(2-oxopyri...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H21ClN4O4S/c1-14(29)27-12-17(18(13-27)26-23(32)19-9-10-20(24)33-19)25-22(31)15-5-7-16(8-6-15)28-11-3-2-4-21(28)30/h2-11,17-18H,12-13H2,1H3,(H,25,31)(H,26,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218283
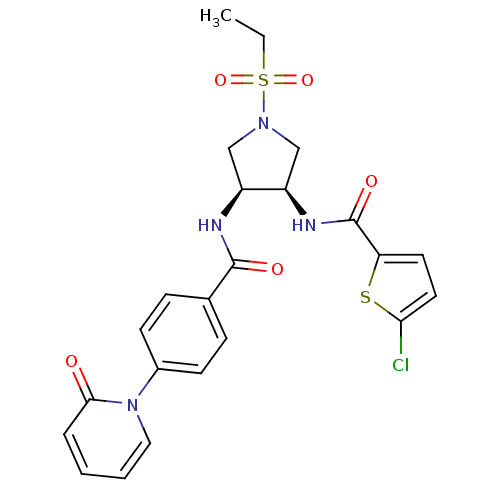
(5-chloro-N-((3R,4S)-1-(ethylsulfonyl)-4-(4-(2-oxop...)Show SMILES CCS(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H23ClN4O5S2/c1-2-35(32,33)27-13-17(18(14-27)26-23(31)19-10-11-20(24)34-19)25-22(30)15-6-8-16(9-7-15)28-12-4-3-5-21(28)29/h3-12,17-18H,2,13-14H2,1H3,(H,25,30)(H,26,31)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50216604

(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H23ClN4O3/c27-20-15-28-23-14-17(9-12-19(20)23)26(34)30-22-5-3-4-21(22)29-25(33)16-7-10-18(11-8-16)31-13-2-1-6-24(31)32/h1-2,6-15,21-22,28H,3-5H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a in presence of tripeptide substrate at 25 degC |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218306
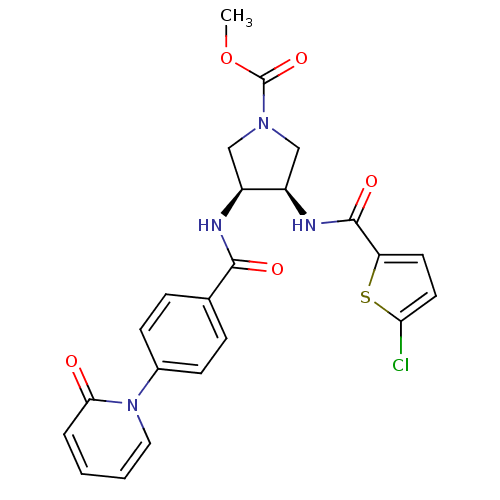
((3R,4S)-methyl 3-(2-chlorothiophene-5-carboxamido)...)Show SMILES COC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H21ClN4O5S/c1-33-23(32)27-12-16(17(13-27)26-22(31)18-9-10-19(24)34-18)25-21(30)14-5-7-15(8-6-14)28-11-3-2-4-20(28)29/h2-11,16-17H,12-13H2,1H3,(H,25,30)(H,26,31)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218319
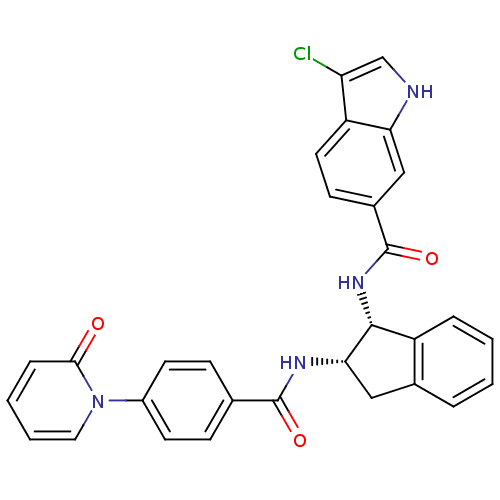
(3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1[C@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)34-28-22-6-2-1-5-19(22)15-26(28)33-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,37)(H,34,38)/t26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218294

((3R,4S)-2-methoxyethyl 3-(2-chlorothiophene-5-carb...)Show SMILES COCCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H25ClN4O6S/c1-35-12-13-36-25(34)29-14-18(19(15-29)28-24(33)20-9-10-21(26)37-20)27-23(32)16-5-7-17(8-6-16)30-11-3-2-4-22(30)31/h2-11,18-19H,12-15H2,1H3,(H,27,32)(H,28,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218301
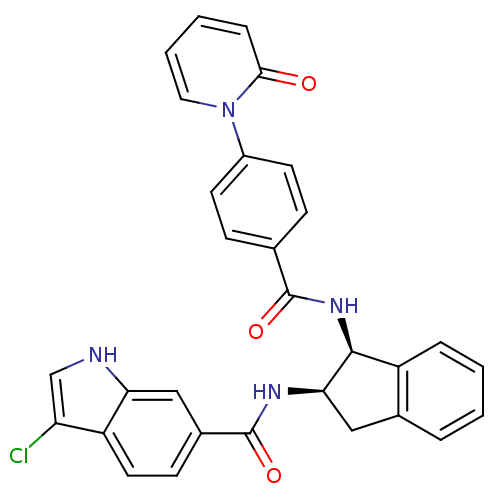
(3-chloro-N-((1S,2R)-1-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1Cc2ccccc2[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)33-26-15-19-5-1-2-6-22(19)28(26)34-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,38)(H,34,37)/t26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218286
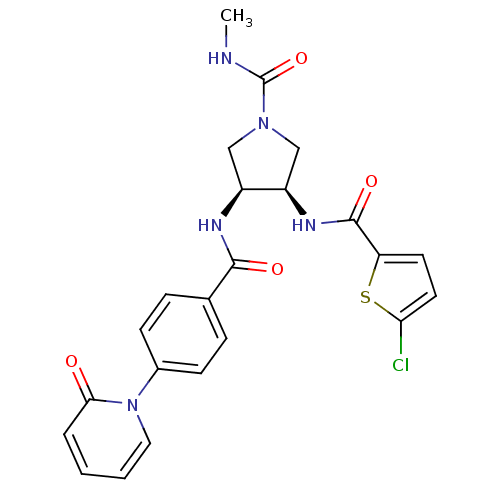
((3R,4S)-3-(2-chlorothiophene-5-carboxamido)-N-meth...)Show SMILES CNC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H22ClN5O4S/c1-25-23(33)28-12-16(17(13-28)27-22(32)18-9-10-19(24)34-18)26-21(31)14-5-7-15(8-6-14)29-11-3-2-4-20(29)30/h2-11,16-17H,12-13H2,1H3,(H,25,33)(H,26,31)(H,27,32)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218295
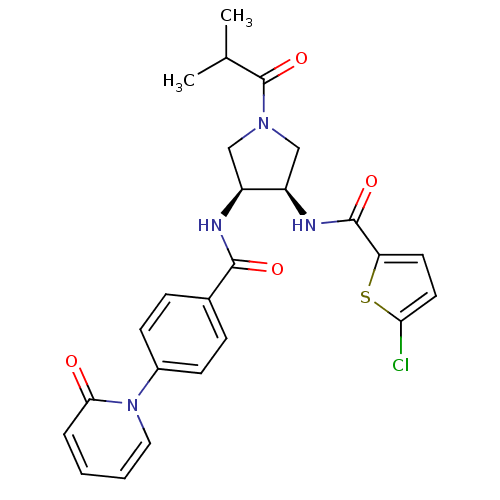
(5-chloro-N-((3R,4S)-1-isobutyryl-4-(4-(2-oxopyridi...)Show SMILES CC(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H25ClN4O4S/c1-15(2)25(34)29-13-18(19(14-29)28-24(33)20-10-11-21(26)35-20)27-23(32)16-6-8-17(9-7-16)30-12-4-3-5-22(30)31/h3-12,15,18-19H,13-14H2,1-2H3,(H,27,32)(H,28,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218314
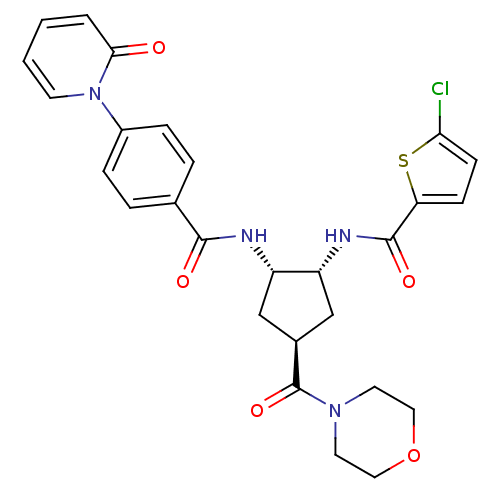
(5-chloro-N-((1R,2S,4S)-4-(morpholine-4-carbonyl)-2...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1C[C@H](C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H27ClN4O5S/c28-23-9-8-22(38-23)26(35)30-21-16-18(27(36)31-11-13-37-14-12-31)15-20(21)29-25(34)17-4-6-19(7-5-17)32-10-2-1-3-24(32)33/h1-10,18,20-21H,11-16H2,(H,29,34)(H,30,35)/t18-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218284
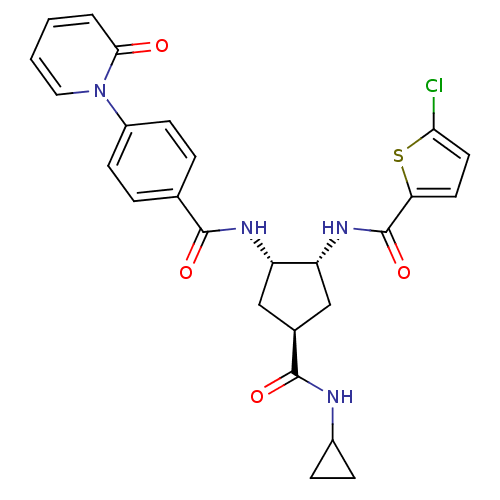
(5-chloro-N-((1R,2S,4S)-4-(cyclopropylcarbamoyl)-2-...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1C[C@H](C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)NC1CC1 Show InChI InChI=1S/C26H25ClN4O4S/c27-22-11-10-21(36-22)26(35)30-20-14-16(25(34)28-17-6-7-17)13-19(20)29-24(33)15-4-8-18(9-5-15)31-12-2-1-3-23(31)32/h1-5,8-12,16-17,19-20H,6-7,13-14H2,(H,28,34)(H,29,33)(H,30,35)/t16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218281
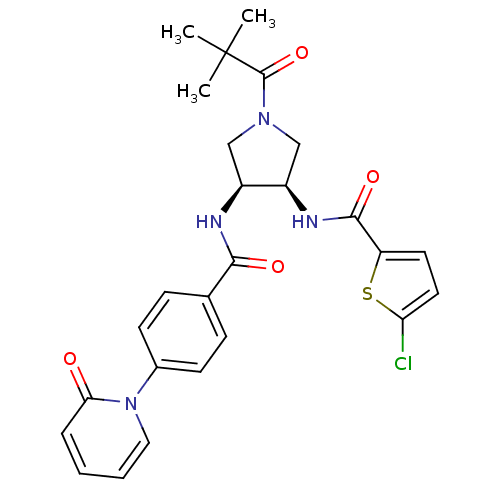
(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES CC(C)(C)C(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H27ClN4O4S/c1-26(2,3)25(35)30-14-18(19(15-30)29-24(34)20-11-12-21(27)36-20)28-23(33)16-7-9-17(10-8-16)31-13-5-4-6-22(31)32/h4-13,18-19H,14-15H2,1-3H3,(H,28,33)(H,29,34)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218304
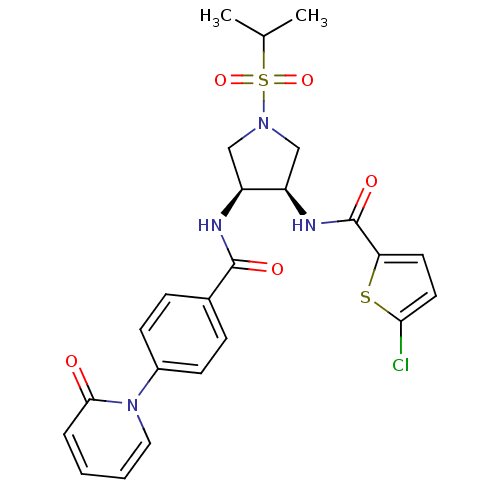
(5-chloro-N-((3R,4S)-1-(isopropylsulfonyl)-4-(4-(2-...)Show SMILES CC(C)S(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H25ClN4O5S2/c1-15(2)36(33,34)28-13-18(19(14-28)27-24(32)20-10-11-21(25)35-20)26-23(31)16-6-8-17(9-7-16)29-12-4-3-5-22(29)30/h3-12,15,18-19H,13-14H2,1-2H3,(H,26,31)(H,27,32)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218296
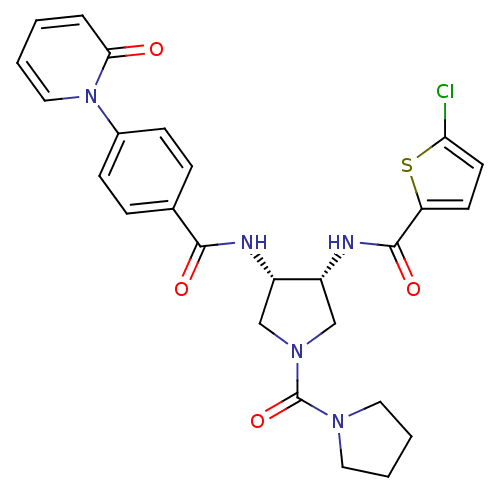
(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)N1CCCC1 Show InChI InChI=1S/C26H26ClN5O4S/c27-22-11-10-21(37-22)25(35)29-20-16-31(26(36)30-12-3-4-13-30)15-19(20)28-24(34)17-6-8-18(9-7-17)32-14-2-1-5-23(32)33/h1-2,5-11,14,19-20H,3-4,12-13,15-16H2,(H,28,34)(H,29,35)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218287

((1S,3R,4S)-3-(2-chlorothiophene-5-carboxamido)-4-(...)Show SMILES OC(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H20ClN3O5S/c24-19-9-8-18(33-19)22(30)26-17-12-14(23(31)32)11-16(17)25-21(29)13-4-6-15(7-5-13)27-10-2-1-3-20(27)28/h1-10,14,16-17H,11-12H2,(H,25,29)(H,26,30)(H,31,32)/t14-,16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218320

(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES CCC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O4S/c1-2-21(30)28-13-17(18(14-28)27-24(33)19-10-11-20(25)34-19)26-23(32)15-6-8-16(9-7-15)29-12-4-3-5-22(29)31/h3-12,17-18H,2,13-14H2,1H3,(H,26,32)(H,27,33)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218310
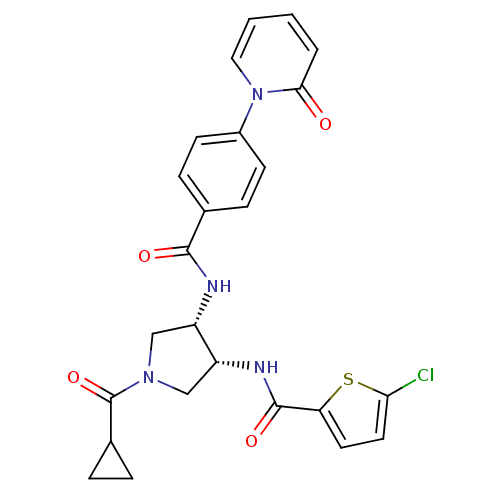
(5-chloro-N-((3R,4S)-1-(cyclopropanecarbonyl)-4-(4-...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)C1CC1 Show InChI InChI=1S/C25H23ClN4O4S/c26-21-11-10-20(35-21)24(33)28-19-14-29(25(34)16-4-5-16)13-18(19)27-23(32)15-6-8-17(9-7-15)30-12-2-1-3-22(30)31/h1-3,6-12,16,18-19H,4-5,13-14H2,(H,27,32)(H,28,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218297
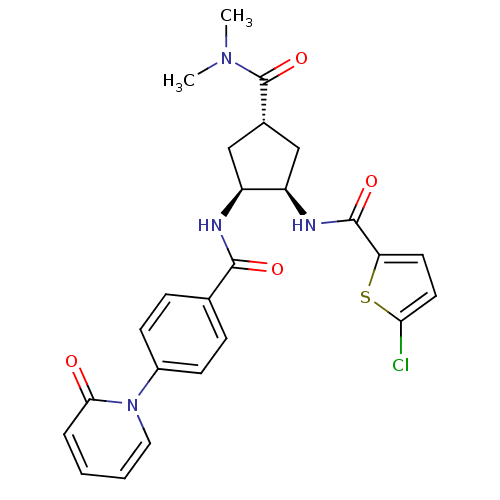
(5-chloro-N-((1R,2S,4S)-4-(dimethylcarbamoyl)-2-(4-...)Show SMILES CN(C)C(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H25ClN4O4S/c1-29(2)25(34)16-13-18(19(14-16)28-24(33)20-10-11-21(26)35-20)27-23(32)15-6-8-17(9-7-15)30-12-4-3-5-22(30)31/h3-12,16,18-19H,13-14H2,1-2H3,(H,27,32)(H,28,33)/t16-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218307

(CHEMBL235150 | N-((3R,4S)-1-benzoyl-4-(4-(2-oxopyr...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)c1ccccc1 Show InChI InChI=1S/C28H23ClN4O4S/c29-24-14-13-23(38-24)27(36)31-22-17-32(28(37)19-6-2-1-3-7-19)16-21(22)30-26(35)18-9-11-20(12-10-18)33-15-5-4-8-25(33)34/h1-15,21-22H,16-17H2,(H,30,35)(H,31,36)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218285

(5-chloro-N-((3S,4R)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1COC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C21H18ClN3O4S/c22-18-9-8-17(30-18)21(28)24-16-12-29-11-15(16)23-20(27)13-4-6-14(7-5-13)25-10-2-1-3-19(25)26/h1-10,15-16H,11-12H2,(H,23,27)(H,24,28)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218318
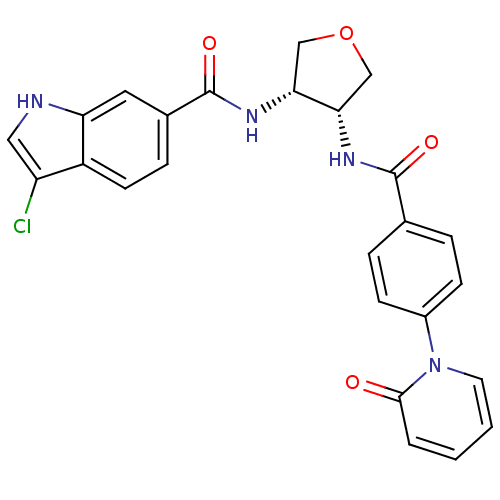
(3-chloro-N-((3S,4R)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1COC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H21ClN4O4/c26-19-12-27-20-11-16(6-9-18(19)20)25(33)29-22-14-34-13-21(22)28-24(32)15-4-7-17(8-5-15)30-10-2-1-3-23(30)31/h1-12,21-22,27H,13-14H2,(H,28,32)(H,29,33)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a in presence of tripeptide substrate at 37 degC |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218317
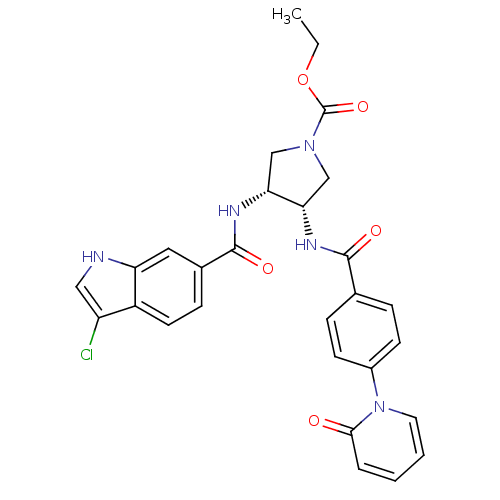
((3R,4S)-ethyl 3-(3-chloro-1H-indole-6-carboxamido)...)Show SMILES CCOC(=O)N1C[C@H](NC(=O)c2ccc(cc2)-n2ccccc2=O)[C@@H](C1)NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C28H26ClN5O5/c1-2-39-28(38)33-15-23(24(16-33)32-27(37)18-8-11-20-21(29)14-30-22(20)13-18)31-26(36)17-6-9-19(10-7-17)34-12-4-3-5-25(34)35/h3-14,23-24,30H,2,15-16H2,1H3,(H,31,36)(H,32,37)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218302
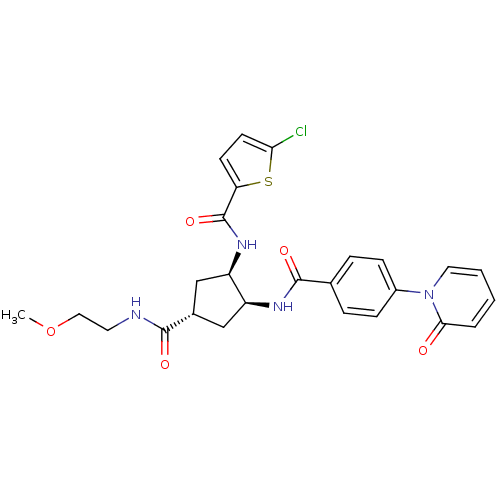
(CHEMBL445941 | N-((1R,2S,4S)-4-((2-methoxyethyl)ca...)Show SMILES COCCNC(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H27ClN4O5S/c1-36-13-11-28-24(33)17-14-19(20(15-17)30-26(35)21-9-10-22(27)37-21)29-25(34)16-5-7-18(8-6-16)31-12-3-2-4-23(31)32/h2-10,12,17,19-20H,11,13-15H2,1H3,(H,28,33)(H,29,34)(H,30,35)/t17-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218299
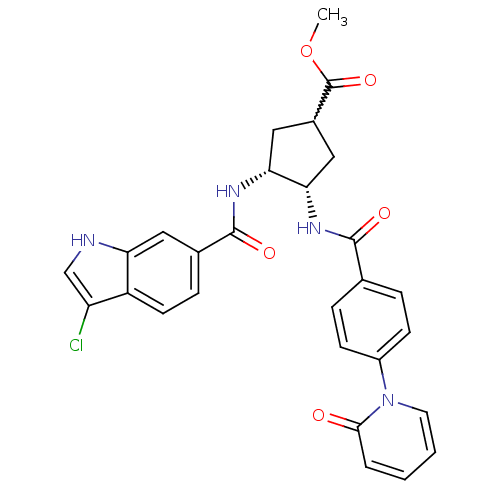
((3R,4S)-methyl 3-(3-chloro-1H-indole-6-carboxamido...)Show SMILES COC(=O)C1C[C@H](NC(=O)c2ccc(cc2)-n2ccccc2=O)[C@@H](C1)NC(=O)c1ccc2c(Cl)c[nH]c2c1 |w:4.3| Show InChI InChI=1S/C28H25ClN4O5/c1-38-28(37)18-13-23(24(14-18)32-27(36)17-7-10-20-21(29)15-30-22(20)12-17)31-26(35)16-5-8-19(9-6-16)33-11-3-2-4-25(33)34/h2-12,15,18,23-24,30H,13-14H2,1H3,(H,31,35)(H,32,36)/t18?,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218292
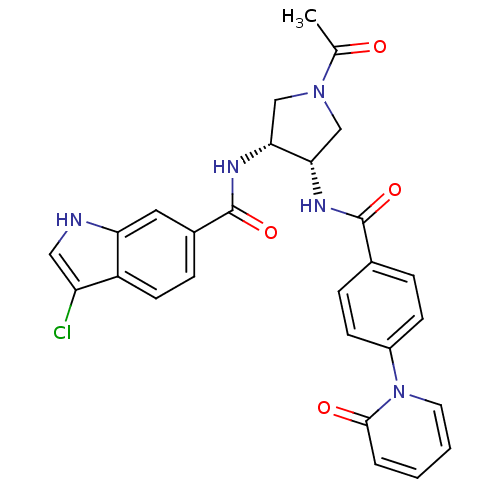
(CHEMBL237924 | N-((3R,4S)-1-acetyl-4-(4-(2-oxopyri...)Show SMILES CC(=O)N1C[C@H](NC(=O)c2ccc(cc2)-n2ccccc2=O)[C@@H](C1)NC(=O)c1ccc2c(Cl)c[nH]c2c1 Show InChI InChI=1S/C27H24ClN5O4/c1-16(34)32-14-23(24(15-32)31-27(37)18-7-10-20-21(28)13-29-22(20)12-18)30-26(36)17-5-8-19(9-6-17)33-11-3-2-4-25(33)35/h2-13,23-24,29H,14-15H2,1H3,(H,30,36)(H,31,37)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218311

((3R,4S)-(9H-fluoren-9-yl)methyl 3-(2-chlorothiophe...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C36H29ClN4O5S/c37-32-17-16-31(47-32)35(44)39-30-20-40(36(45)46-21-28-26-9-3-1-7-24(26)25-8-2-4-10-27(25)28)19-29(30)38-34(43)22-12-14-23(15-13-22)41-18-6-5-11-33(41)42/h1-18,28-30H,19-21H2,(H,38,43)(H,39,44)/t29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a in presence of prothrombin at 25 degC |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218321
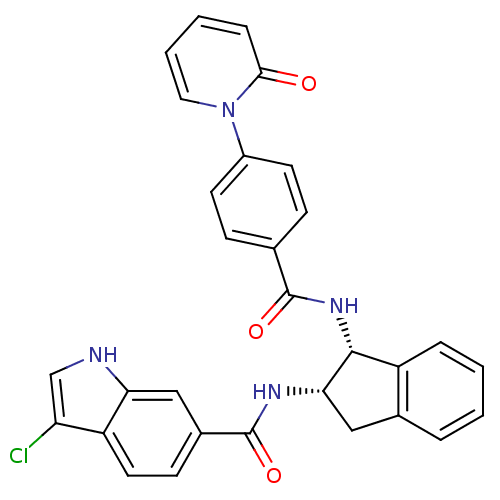
(3-chloro-N-((1R,2S)-1-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@H]1Cc2ccccc2[C@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C30H23ClN4O3/c31-24-17-32-25-16-20(10-13-23(24)25)30(38)33-26-15-19-5-1-2-6-22(19)28(26)34-29(37)18-8-11-21(12-9-18)35-14-4-3-7-27(35)36/h1-14,16-17,26,28,32H,15H2,(H,33,38)(H,34,37)/t26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218288

(5-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1CNC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C21H19ClN4O3S/c22-18-9-8-17(30-18)21(29)25-16-12-23-11-15(16)24-20(28)13-4-6-14(7-5-13)26-10-2-1-3-19(26)27/h1-10,15-16,23H,11-12H2,(H,24,28)(H,25,29)/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218293
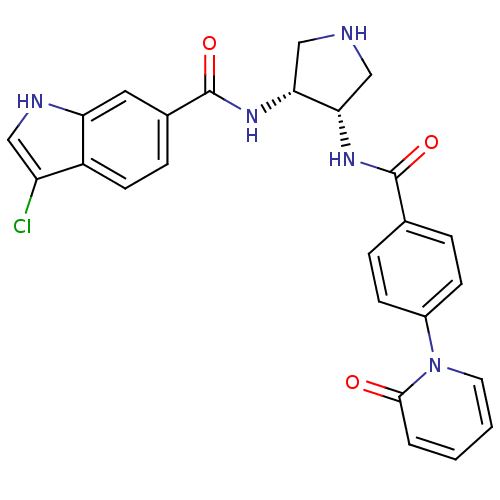
(3-chloro-N-((3R,4S)-4-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CNC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H22ClN5O3/c26-19-12-28-20-11-16(6-9-18(19)20)25(34)30-22-14-27-13-21(22)29-24(33)15-4-7-17(8-5-15)31-10-2-1-3-23(31)32/h1-12,21-22,27-28H,13-14H2,(H,29,33)(H,30,34)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218303
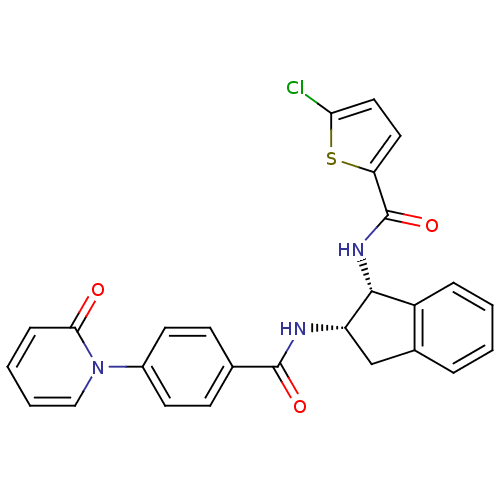
(5-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@H]1[C@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H20ClN3O3S/c27-22-13-12-21(34-22)26(33)29-24-19-6-2-1-5-17(19)15-20(24)28-25(32)16-8-10-18(11-9-16)30-14-4-3-7-23(30)31/h1-14,20,24H,15H2,(H,28,32)(H,29,33)/t20-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a in presence of saturating prothrombin at 37 degC |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218309
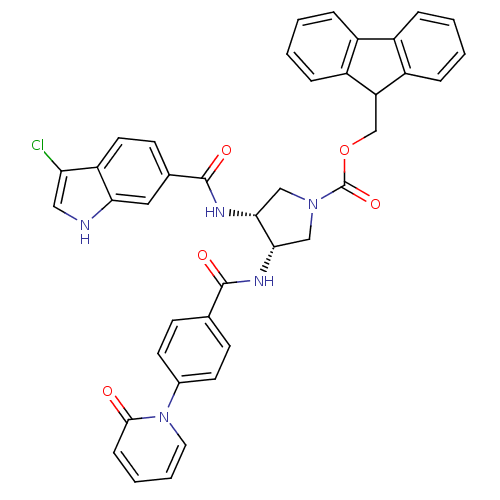
((3R,4S)-(9H-fluoren-9-yl)methyl 3-(3-chloro-1H-ind...)Show SMILES Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CN(C[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O)C(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C40H32ClN5O5/c41-33-20-42-34-19-25(14-17-31(33)34)39(49)44-36-22-45(40(50)51-23-32-29-9-3-1-7-27(29)28-8-2-4-10-30(28)32)21-35(36)43-38(48)24-12-15-26(16-13-24)46-18-6-5-11-37(46)47/h1-20,32,35-36,42H,21-23H2,(H,43,48)(H,44,49)/t35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50218289

(5-chloro-N-((1S,2R)-2-(4-(2-oxopyridin-1(2H)-yl)be...)Show SMILES Clc1ccc(s1)C(=O)N[C@@H]1[C@@H](Cc2ccccc12)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C26H20ClN3O3S/c27-22-13-12-21(34-22)26(33)29-24-19-6-2-1-5-17(19)15-20(24)28-25(32)16-8-10-18(11-9-16)30-14-4-3-7-23(30)31/h1-14,20,24H,15H2,(H,28,32)(H,29,33)/t20-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50218283

(5-chloro-N-((3R,4S)-1-(ethylsulfonyl)-4-(4-(2-oxop...)Show SMILES CCS(=O)(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H23ClN4O5S2/c1-2-35(32,33)27-13-17(18(14-27)26-23(31)19-10-11-20(24)34-19)25-22(30)15-6-8-16(9-7-15)28-12-4-3-5-21(28)29/h3-12,17-18H,2,13-14H2,1H3,(H,25,30)(H,26,31)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50218282

((3R,4S)-ethyl 3-(2-chlorothiophene-5-carboxamido)-...)Show SMILES CCOC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C24H23ClN4O5S/c1-2-34-24(33)28-13-17(18(14-28)27-23(32)19-10-11-20(25)35-19)26-22(31)15-6-8-16(9-7-15)29-12-4-3-5-21(29)30/h3-12,17-18H,2,13-14H2,1H3,(H,26,31)(H,27,32)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50218291

(CHEMBL394129 | N-((3R,4S)-1-acetyl-4-(4-(2-oxopyri...)Show SMILES CC(=O)N1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H21ClN4O4S/c1-14(29)27-12-17(18(13-27)26-23(32)19-9-10-20(24)33-19)25-22(31)15-5-7-16(8-6-15)28-11-3-2-4-21(28)30/h2-11,17-18H,12-13H2,1H3,(H,25,31)(H,26,32)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50218287

((1S,3R,4S)-3-(2-chlorothiophene-5-carboxamido)-4-(...)Show SMILES OC(=O)[C@@H]1C[C@@H](NC(=O)c2ccc(Cl)s2)[C@H](C1)NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C23H20ClN3O5S/c24-19-9-8-18(33-19)22(30)26-17-12-14(23(31)32)11-16(17)25-21(29)13-4-6-15(7-5-13)27-10-2-1-3-20(27)28/h1-10,14,16-17H,11-12H2,(H,25,29)(H,26,30)(H,31,32)/t14-,16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein |
Bioorg Med Chem Lett 17: 5041-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.020
BindingDB Entry DOI: 10.7270/Q23J3CPQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data