

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111776 (CHEMBL3605361) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111774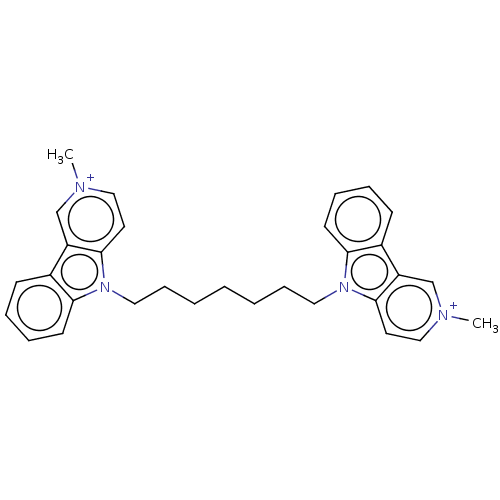 (CHEMBL3605359) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317179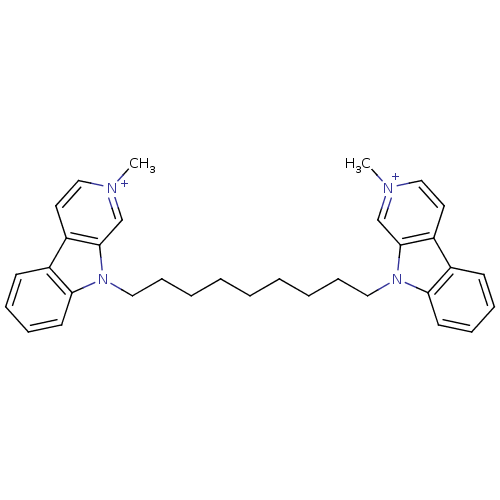 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111775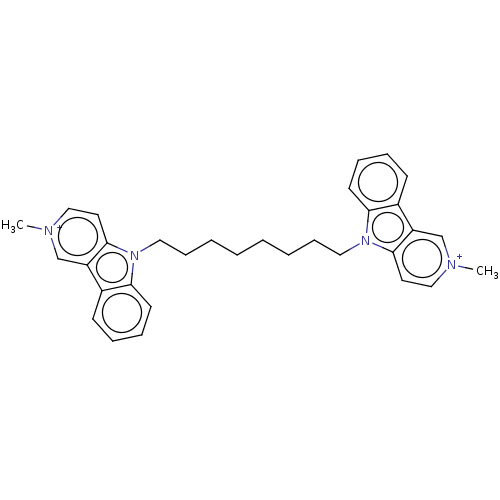 (CHEMBL3605360) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111778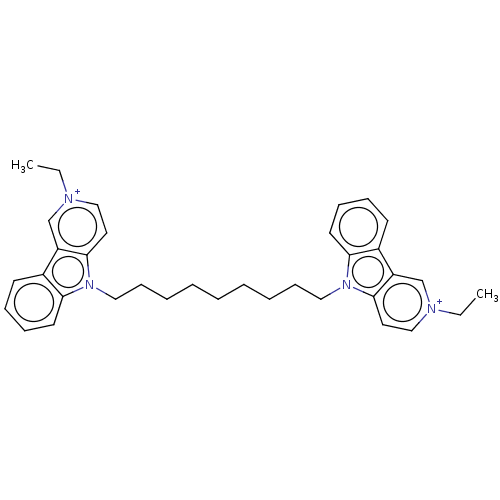 (CHEMBL3605363) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111780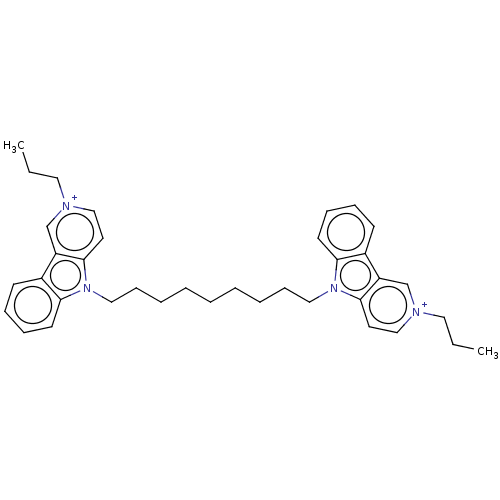 (CHEMBL3605365) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111777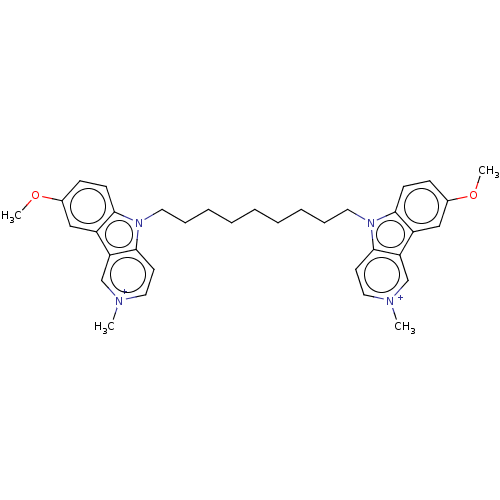 (CHEMBL3605362) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317179 (2-Methyl-9-[9-(2-methyl-beta-carboline-9-yl)nonyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111772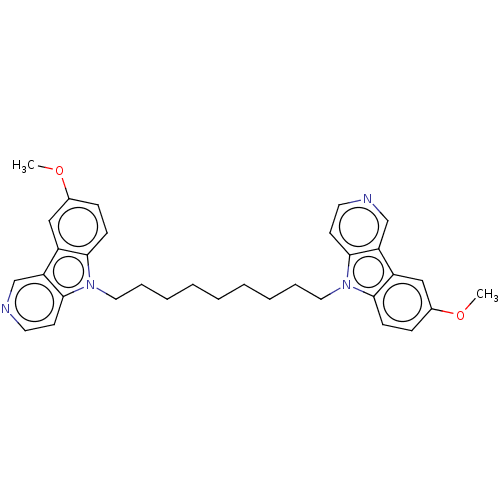 (CHEMBL3605357) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111779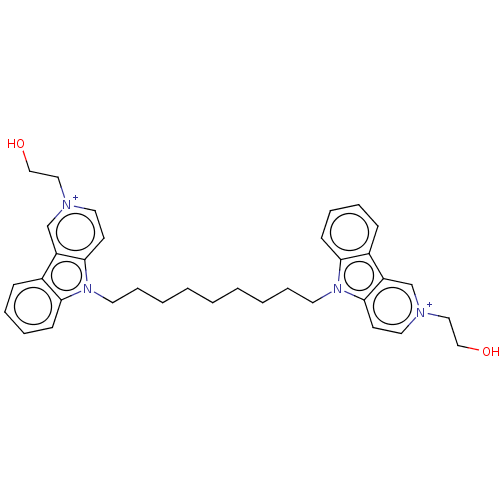 (CHEMBL3605364) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111785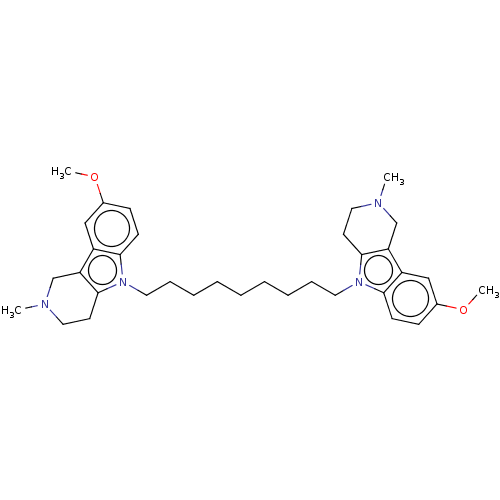 (CHEMBL3605370) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111770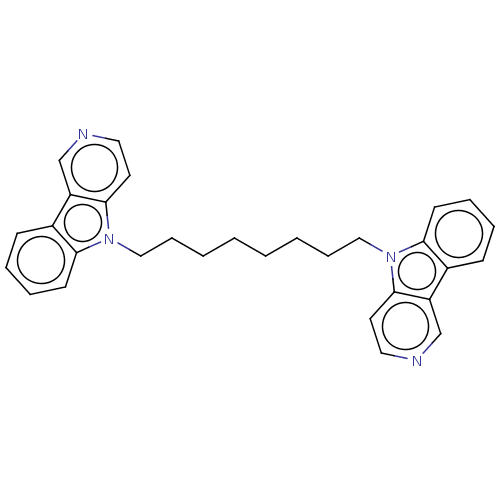 (CHEMBL3605355) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111772 (CHEMBL3605357) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111771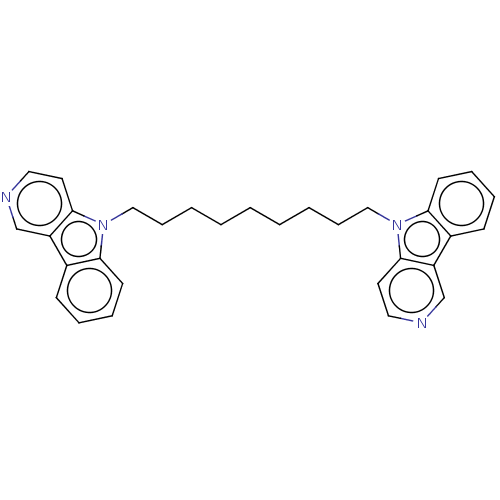 (CHEMBL3605356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111773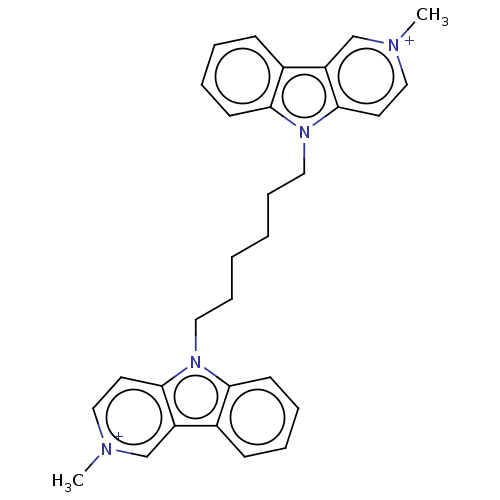 (CHEMBL3605358) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111770 (CHEMBL3605355) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111776 (CHEMBL3605361) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111769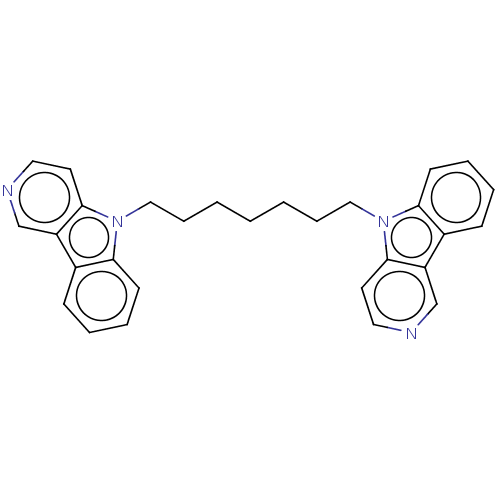 (CHEMBL3605354) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317193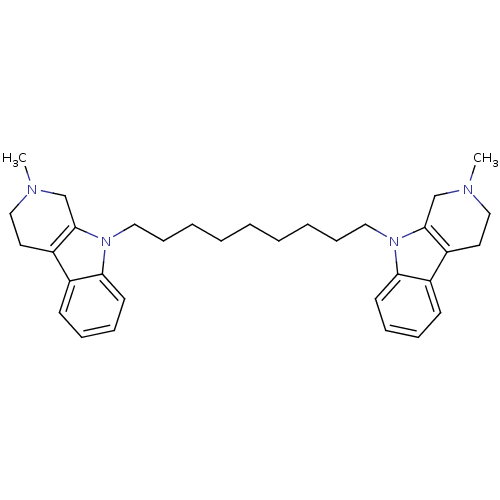 (2-Methyl-9-[9-(2-methyl-1,2,3,4-tetrahydro-beta-ca...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111784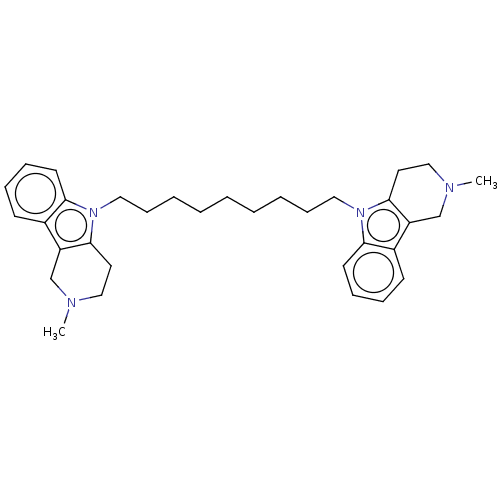 (CHEMBL3605369) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317193 (2-Methyl-9-[9-(2-methyl-1,2,3,4-tetrahydro-beta-ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111775 (CHEMBL3605360) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111782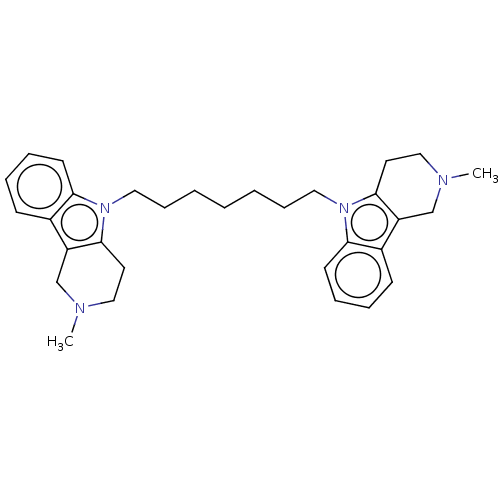 (CHEMBL3605367) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111778 (CHEMBL3605363) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111783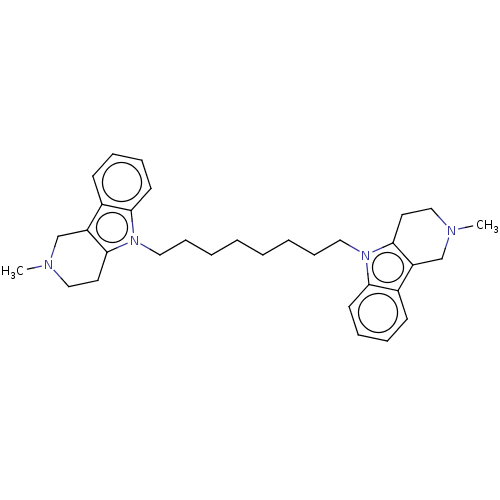 (CHEMBL3605368) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111782 (CHEMBL3605367) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111771 (CHEMBL3605356) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111783 (CHEMBL3605368) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111785 (CHEMBL3605370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111774 (CHEMBL3605359) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111777 (CHEMBL3605362) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111768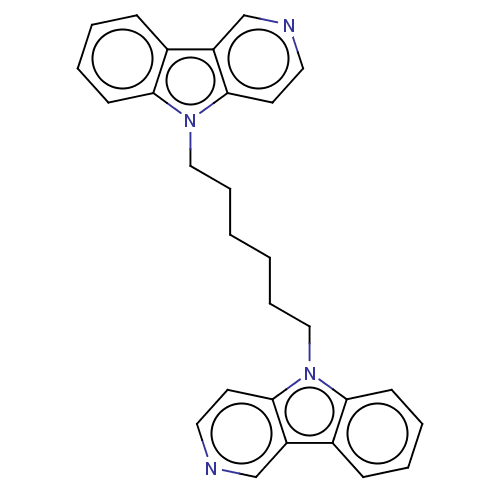 (CHEMBL3605353) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50111774 (CHEMBL3605359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111784 (CHEMBL3605369) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111780 (CHEMBL3605365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50111776 (CHEMBL3605361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50111775 (CHEMBL3605360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111781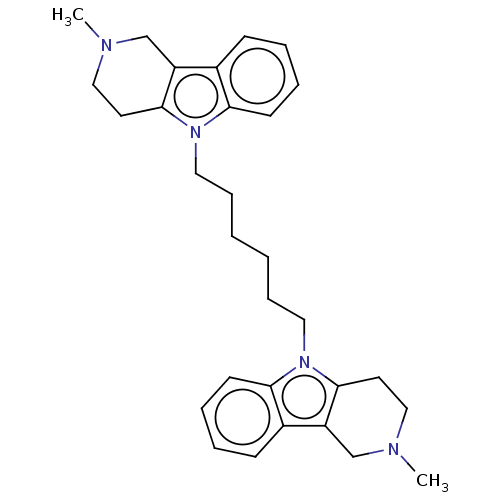 (CHEMBL3605366) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111781 (CHEMBL3605366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111779 (CHEMBL3605364) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50111769 (CHEMBL3605354) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50111773 (CHEMBL3605358) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of BChE in equine serum using butyrylthiocholine iodide substrate by Ellman assay | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50111773 (CHEMBL3605358) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50111774 (CHEMBL3605359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50111776 (CHEMBL3605361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50111775 (CHEMBL3605360) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jena Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB | J Med Chem 58: 6710-5 (2015) Article DOI: 10.1021/acs.jmedchem.5b00958 BindingDB Entry DOI: 10.7270/Q2QZ2CRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |