 Found 112 hits Enz. Inhib. hit(s) with all data for entry = 50022018
Found 112 hits Enz. Inhib. hit(s) with all data for entry = 50022018 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11551
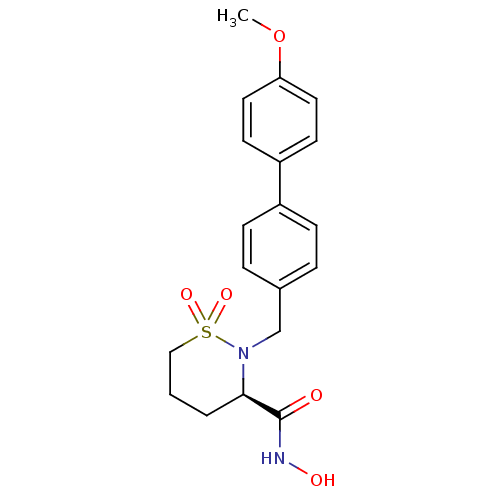
((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50160855
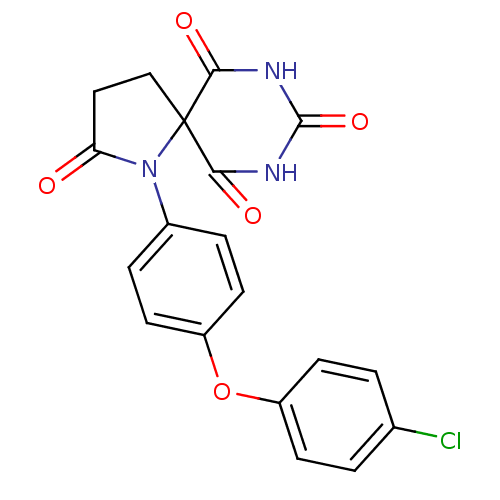
(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50160855

(1-[4-(4-chloro-phenoxy)-phenyl]-1,7,9-triaza-spiro...)Show SMILES Clc1ccc(Oc2ccc(cc2)N2C(=O)CCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C19H14ClN3O5/c20-11-1-5-13(6-2-11)28-14-7-3-12(4-8-14)23-15(24)9-10-19(23)16(25)21-18(27)22-17(19)26/h1-8H,9-10H2,(H2,21,22,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM11551

((3R)-N-hydroxy-2-[(4-methoxy-1,1-biphenyl-4-yl)met...)Show SMILES COc1ccc(cc1)-c1ccc(CN2[C@H](CCCS2(=O)=O)C(=O)NO)cc1 |r| Show InChI InChI=1S/C19H22N2O5S/c1-26-17-10-8-16(9-11-17)15-6-4-14(5-7-15)13-21-18(19(22)20-23)3-2-12-27(21,24)25/h4-11,18,23H,2-3,12-13H2,1H3,(H,20,22)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230525

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-2-ylsul...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(Cl)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C22H28ClN3O6S/c1-15-12-25(13-16(2)31-15)21(22(27)24-28)14-26(33(3,29)30)18-6-10-20(11-7-18)32-19-8-4-17(23)5-9-19/h4-11,15-16,21,28H,12-14H2,1-3H3,(H,24,27)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230526

((S)-N-hydroxy-2-(isopropylamino)-3-(N-(4-(p-tolylo...)Show SMILES CC(C)N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C20H27N3O5S/c1-14(2)21-19(20(24)22-25)13-23(29(4,26)27)16-7-11-18(12-8-16)28-17-9-5-15(3)6-10-17/h5-12,14,19,21,25H,13H2,1-4H3,(H,22,24)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230497
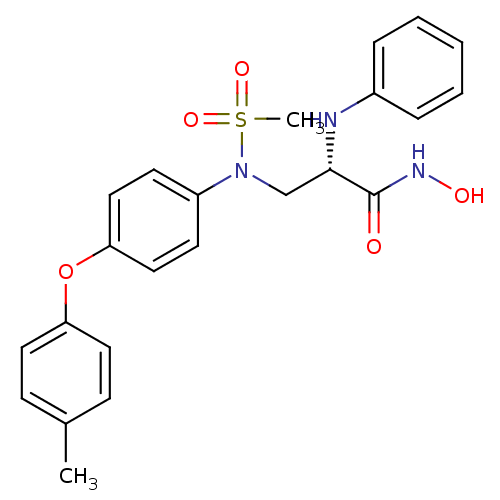
((S)-N-hydroxy-2-(phenylamino)-3-(N-(4-(p-tolyloxy)...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2ccccc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H25N3O5S/c1-17-8-12-20(13-9-17)31-21-14-10-19(11-15-21)26(32(2,29)30)16-22(23(27)25-28)24-18-6-4-3-5-7-18/h3-15,22,24,28H,16H2,1-2H3,(H,25,27)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230508

((S)-N-hydroxy-2-((S)-1-phenylethylamino)-3-(N-(4-(...)Show SMILES C[C@H](N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO)c1ccccc1 Show InChI InChI=1S/C25H29N3O5S/c1-18-9-13-22(14-10-18)33-23-15-11-21(12-16-23)28(34(3,31)32)17-24(25(29)27-30)26-19(2)20-7-5-4-6-8-20/h4-16,19,24,26,30H,17H2,1-3H3,(H,27,29)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230496

((S)-N-hydroxy-2-(3-methoxyphenylamino)-3-(N-(4-(p-...)Show SMILES COc1cccc(N[C@@H](CN(c2ccc(Oc3ccc(C)cc3)cc2)S(C)(=O)=O)C(=O)NO)c1 Show InChI InChI=1S/C24H27N3O6S/c1-17-7-11-20(12-8-17)33-21-13-9-19(10-14-21)27(34(3,30)31)16-23(24(28)26-29)25-18-5-4-6-22(15-18)32-2/h4-15,23,25,29H,16H2,1-3H3,(H,26,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230512
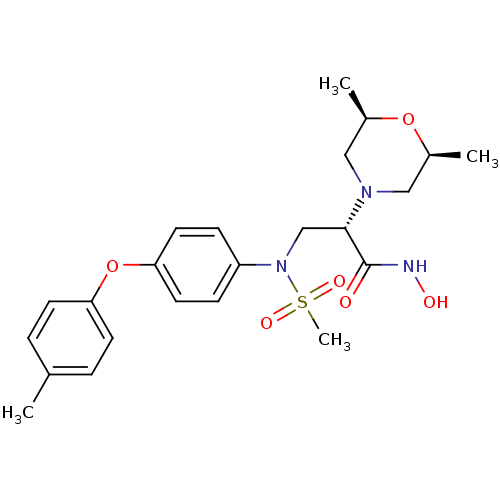
((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-5-9-20(10-6-16)32-21-11-7-19(8-12-21)26(33(4,29)30)15-22(23(27)24-28)25-13-17(2)31-18(3)14-25/h5-12,17-18,22,28H,13-15H2,1-4H3,(H,24,27)/t17-,18+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230507

((S)-2-(3-fluorophenylamino)-N-hydroxy-3-(N-(4-(p-t...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2cccc(F)c2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H24FN3O5S/c1-16-6-10-20(11-7-16)32-21-12-8-19(9-13-21)27(33(2,30)31)15-22(23(28)26-29)25-18-5-3-4-17(24)14-18/h3-14,22,25,29H,15H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230518

((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H28F3N3O6S/c1-15-12-28(13-16(2)34-15)21(22(30)27-31)14-29(36(3,32)33)18-6-10-20(11-7-18)35-19-8-4-17(5-9-19)23(24,25)26/h4-11,15-16,21,31H,12-14H2,1-3H3,(H,27,30)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230516
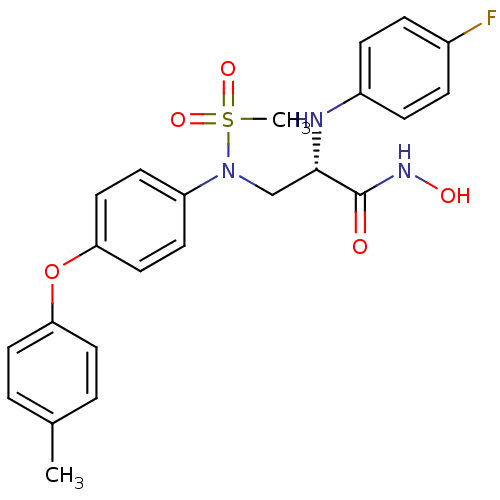
((S)-2-(4-fluorophenylamino)-N-hydroxy-3-(N-(4-(p-t...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](Nc2ccc(F)cc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H24FN3O5S/c1-16-3-11-20(12-4-16)32-21-13-9-19(10-14-21)27(33(2,30)31)15-22(23(28)26-29)25-18-7-5-17(24)6-8-18/h3-14,22,25,29H,15H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230499

((S)-N-hydroxy-2-(isobutylamino)-3-(N-(4-(p-tolylox...)Show SMILES CC(C)CN[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C21H29N3O5S/c1-15(2)13-22-20(21(25)23-26)14-24(30(4,27)28)17-7-11-19(12-8-17)29-18-9-5-16(3)6-10-18/h5-12,15,20,22,26H,13-14H2,1-4H3,(H,23,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230521

((S)-N-hydroxy-2-((R)-1-phenylethylamino)-3-(N-(4-(...)Show SMILES C[C@@H](N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO)c1ccccc1 Show InChI InChI=1S/C25H29N3O5S/c1-18-9-13-22(14-10-18)33-23-15-11-21(12-16-23)28(34(3,31)32)17-24(25(29)27-30)26-19(2)20-7-5-4-6-8-20/h4-16,19,24,26,30H,17H2,1-3H3,(H,27,29)/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230524
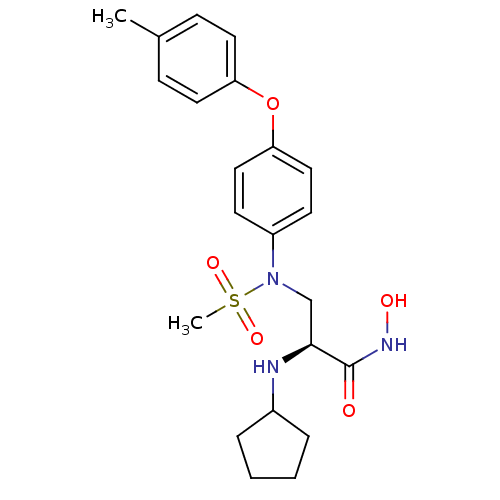
((S)-2-(cyclopentylamino)-N-hydroxy-3-(N-(4-(p-toly...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](NC2CCCC2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C22H29N3O5S/c1-16-7-11-19(12-8-16)30-20-13-9-18(10-14-20)25(31(2,28)29)15-21(22(26)24-27)23-17-5-3-4-6-17/h7-14,17,21,23,27H,3-6,15H2,1-2H3,(H,24,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230515

((S)-2-(1,1-dioxo-1lambda*6*-thiomorpholin-4-yl)-N-...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCS(=O)(=O)CC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H24F3N3O7S2/c1-35(30,31)27(14-19(20(28)25-29)26-10-12-36(32,33)13-11-26)16-4-8-18(9-5-16)34-17-6-2-15(3-7-17)21(22,23)24/h2-9,19,29H,10-14H2,1H3,(H,25,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230505

((S)-N-hydroxy-2-(pyridin-4-ylmethylamino)-3-(N-(4-...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](NCc2ccncc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H26N4O5S/c1-17-3-7-20(8-4-17)32-21-9-5-19(6-10-21)27(33(2,30)31)16-22(23(28)26-29)25-15-18-11-13-24-14-12-18/h3-14,22,25,29H,15-16H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230528
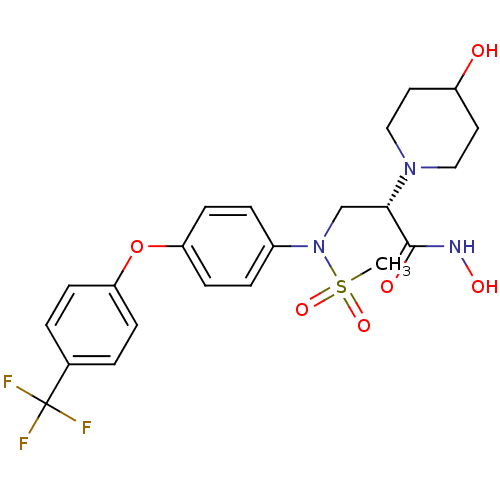
((S)-N-hydroxy-2-(4-hydroxypiperidin-1-yl)-3-(N-(4-...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCC(O)CC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H26F3N3O6S/c1-35(32,33)28(14-20(21(30)26-31)27-12-10-17(29)11-13-27)16-4-8-19(9-5-16)34-18-6-2-15(3-7-18)22(23,24)25/h2-9,17,20,29,31H,10-14H2,1H3,(H,26,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230531

((S)-N-hydroxy-2-morpholino-3-(N-(4-(p-tolyloxy)phe...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](N2CCOCC2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C21H27N3O6S/c1-16-3-7-18(8-4-16)30-19-9-5-17(6-10-19)24(31(2,27)28)15-20(21(25)22-26)23-11-13-29-14-12-23/h3-10,20,26H,11-15H2,1-2H3,(H,22,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230522

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-2-ylsul...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCOCC1)C(=O)NO)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H24ClN3O6S/c1-31(27,28)24(14-19(20(25)22-26)23-10-12-29-13-11-23)16-4-8-18(9-5-16)30-17-6-2-15(21)3-7-17/h2-9,19,26H,10-14H2,1H3,(H,22,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230523
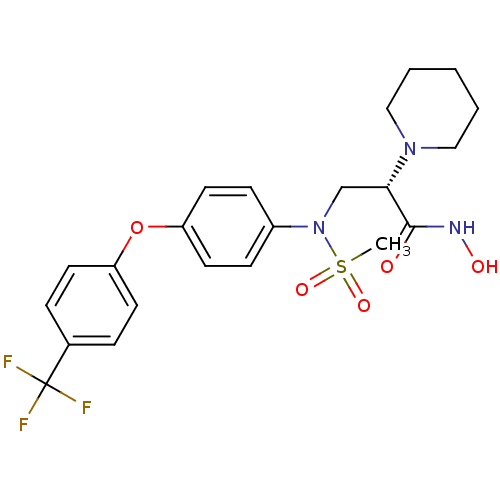
((S)-N-hydroxy-2-(piperidin-1-yl)-3-(N-(4-(4-(trifl...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCCCC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H26F3N3O5S/c1-34(31,32)28(15-20(21(29)26-30)27-13-3-2-4-14-27)17-7-11-19(12-8-17)33-18-9-5-16(6-10-18)22(23,24)25/h5-12,20,30H,2-4,13-15H2,1H3,(H,26,29)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230506

((S)-N-hydroxy-2-(isopropylamino)-3-(N-(4-(4-(trifl...)Show SMILES CC(C)N[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C20H24F3N3O5S/c1-13(2)24-18(19(27)25-28)12-26(32(3,29)30)15-6-10-17(11-7-15)31-16-8-4-14(5-9-16)20(21,22)23/h4-11,13,18,24,28H,12H2,1-3H3,(H,25,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230501

((S)-1-(1-(hydroxyamino)-1-oxo-3-(N-(4-(4-(trifluor...)Show SMILES CNC(=O)C1CCN(CC1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C24H29F3N4O6S/c1-28-22(32)16-11-13-30(14-12-16)21(23(33)29-34)15-31(38(2,35)36)18-5-9-20(10-6-18)37-19-7-3-17(4-8-19)24(25,26)27/h3-10,16,21,34H,11-15H2,1-2H3,(H,28,32)(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230525

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-2-ylsul...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(Cl)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C22H28ClN3O6S/c1-15-12-25(13-16(2)31-15)21(22(27)24-28)14-26(33(3,29)30)18-6-10-20(11-7-18)32-19-8-4-17(23)5-9-19/h4-11,15-16,21,28H,12-14H2,1-3H3,(H,24,27)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230512

((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-5-9-20(10-6-16)32-21-11-7-19(8-12-21)26(33(4,29)30)15-22(23(27)24-28)25-13-17(2)31-18(3)14-25/h5-12,17-18,22,28H,13-15H2,1-4H3,(H,24,27)/t17-,18+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230519

((S)-N-hydroxy-2-(pyridin-3-ylmethylamino)-3-(N-(4-...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](NCc2cccnc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H26N4O5S/c1-17-5-9-20(10-6-17)32-21-11-7-19(8-12-21)27(33(2,30)31)16-22(23(28)26-29)25-15-18-4-3-13-24-14-18/h3-14,22,25,29H,15-16H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230514
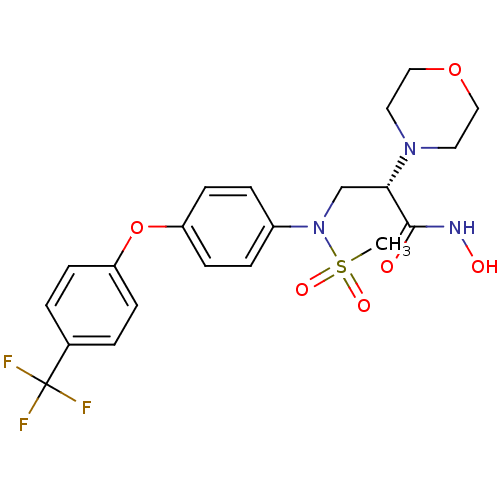
((S)-N-hydroxy-2-morpholino-3-(N-(4-(4-(trifluorome...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCOCC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H24F3N3O6S/c1-34(30,31)27(14-19(20(28)25-29)26-10-12-32-13-11-26)16-4-8-18(9-5-16)33-17-6-2-15(3-7-17)21(22,23)24/h2-9,19,29H,10-14H2,1H3,(H,25,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230498
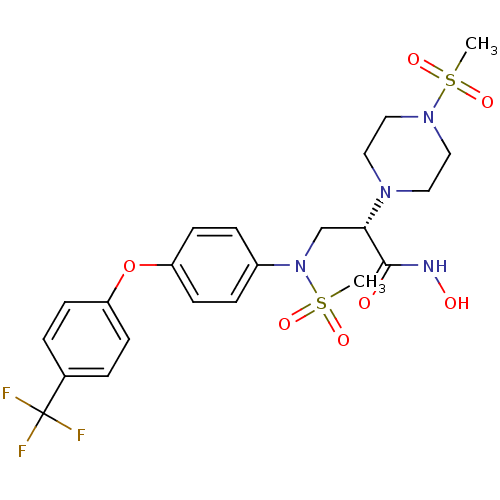
((S)-N-hydroxy-2-(4-(methylsulfonyl)piperazin-1-yl)...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCN(CC1)S(C)(=O)=O)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H27F3N4O7S2/c1-37(32,33)28-13-11-27(12-14-28)20(21(30)26-31)15-29(38(2,34)35)17-5-9-19(10-6-17)36-18-7-3-16(4-8-18)22(23,24)25/h3-10,20,31H,11-15H2,1-2H3,(H,26,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230526

((S)-N-hydroxy-2-(isopropylamino)-3-(N-(4-(p-tolylo...)Show SMILES CC(C)N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C20H27N3O5S/c1-14(2)21-19(20(24)22-25)13-23(29(4,26)27)16-7-11-18(12-8-16)28-17-9-5-15(3)6-10-17/h5-12,14,19,21,25H,13H2,1-4H3,(H,22,24)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230534

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-15-ylsu...)Show SMILES CS(=O)(=O)N(C[C@H](O)C(=O)NO)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C16H17ClN2O6S/c1-26(23,24)19(10-15(20)16(21)18-22)12-4-8-14(9-5-12)25-13-6-2-11(17)3-7-13/h2-9,15,20,22H,10H2,1H3,(H,18,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230515

((S)-2-(1,1-dioxo-1lambda*6*-thiomorpholin-4-yl)-N-...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCS(=O)(=O)CC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H24F3N3O7S2/c1-35(30,31)27(14-19(20(28)25-29)26-10-12-36(32,33)13-11-26)16-4-8-18(9-5-16)34-17-6-2-15(3-7-17)21(22,23)24/h2-9,19,29H,10-14H2,1H3,(H,25,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230533

((S)-2-(4-acetylpiperazin-1-yl)-N-hydroxy-3-(N-(4-(...)Show SMILES CC(=O)N1CCN(CC1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H27F3N4O6S/c1-16(31)28-11-13-29(14-12-28)21(22(32)27-33)15-30(37(2,34)35)18-5-9-20(10-6-18)36-19-7-3-17(4-8-19)23(24,25)26/h3-10,21,33H,11-15H2,1-2H3,(H,27,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230531

((S)-N-hydroxy-2-morpholino-3-(N-(4-(p-tolyloxy)phe...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](N2CCOCC2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C21H27N3O6S/c1-16-3-7-18(8-4-16)30-19-9-5-17(6-10-19)24(31(2,27)28)15-20(21(25)22-26)23-11-13-29-14-12-23/h3-10,20,26H,11-15H2,1-2H3,(H,22,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230510

(CHEMBL252022 | N-{(S)-1-hydroxycarbamoyl-2-[methan...)Show SMILES CC(C)C(=O)N[C@@H](CN(c1ccc(Oc2ccc(C)cc2)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C21H27N3O6S/c1-14(2)20(25)22-19(21(26)23-27)13-24(31(4,28)29)16-7-11-18(12-8-16)30-17-9-5-15(3)6-10-17/h5-12,14,19,27H,13H2,1-4H3,(H,22,25)(H,23,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230501

((S)-1-(1-(hydroxyamino)-1-oxo-3-(N-(4-(4-(trifluor...)Show SMILES CNC(=O)C1CCN(CC1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C24H29F3N4O6S/c1-28-22(32)16-11-13-30(14-12-16)21(23(33)29-34)15-31(38(2,35)36)18-5-9-20(10-6-18)37-19-7-3-17(4-8-19)24(25,26)27/h3-10,16,21,34H,11-15H2,1-2H3,(H,28,32)(H,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230522

((S)-3-(N-(4-(4-chlorophenoxy)phenyl)methan-2-ylsul...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCOCC1)C(=O)NO)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H24ClN3O6S/c1-31(27,28)24(14-19(20(25)22-26)23-10-12-29-13-11-23)16-4-8-18(9-5-16)30-17-6-2-15(21)3-7-17/h2-9,19,26H,10-14H2,1H3,(H,22,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230518

((S)-2-(cis-2,6-dimethylmorpholino)-N-hydroxy-3-(N-...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H28F3N3O6S/c1-15-12-28(13-16(2)34-15)21(22(30)27-31)14-29(36(3,32)33)18-6-10-20(11-7-18)35-19-8-4-17(5-9-19)23(24,25)26/h4-11,15-16,21,31H,12-14H2,1-3H3,(H,27,30)/t15-,16+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230528

((S)-N-hydroxy-2-(4-hydroxypiperidin-1-yl)-3-(N-(4-...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCC(O)CC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H26F3N3O6S/c1-35(32,33)28(14-20(21(30)26-31)27-12-10-17(29)11-13-27)16-4-8-19(9-5-16)34-18-6-2-15(3-7-18)22(23,24)25/h2-9,17,20,29,31H,10-14H2,1H3,(H,26,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230530
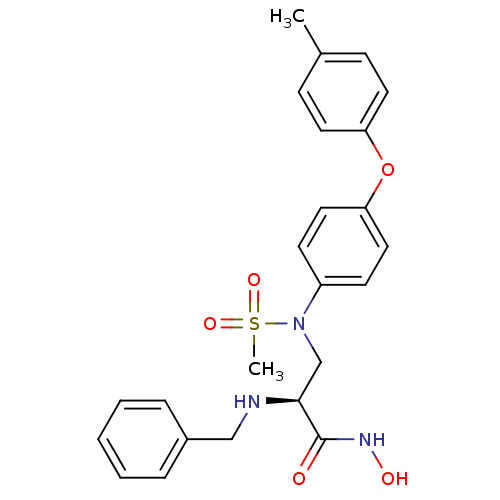
((S)-2-(benzylamino)-N-hydroxy-3-(N-(4-(p-tolyloxy)...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](NCc2ccccc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C24H27N3O5S/c1-18-8-12-21(13-9-18)32-22-14-10-20(11-15-22)27(33(2,30)31)17-23(24(28)26-29)25-16-19-6-4-3-5-7-19/h3-15,23,25,29H,16-17H2,1-2H3,(H,26,28)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230523

((S)-N-hydroxy-2-(piperidin-1-yl)-3-(N-(4-(4-(trifl...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCCCC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H26F3N3O5S/c1-34(31,32)28(15-20(21(29)26-30)27-13-3-2-4-14-27)17-7-11-19(12-8-17)33-18-9-5-16(6-10-18)22(23,24)25/h5-12,20,30H,2-4,13-15H2,1H3,(H,26,29)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230517

((S)-N,2-dihydroxy-3-(N-(4-(p-tolyloxy)phenyl)metha...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](O)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C17H20N2O6S/c1-12-3-7-14(8-4-12)25-15-9-5-13(6-10-15)19(26(2,23)24)11-16(20)17(21)18-22/h3-10,16,20,22H,11H2,1-2H3,(H,18,21)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230533

((S)-2-(4-acetylpiperazin-1-yl)-N-hydroxy-3-(N-(4-(...)Show SMILES CC(=O)N1CCN(CC1)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C23H27F3N4O6S/c1-16(31)28-11-13-29(14-12-28)21(22(32)27-33)15-30(37(2,34)35)18-5-9-20(10-6-18)36-19-7-3-17(4-8-19)23(24,25)26/h3-10,21,33H,11-15H2,1-2H3,(H,27,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50230532
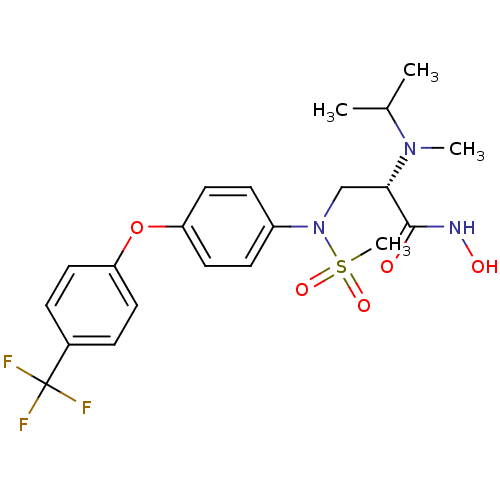
((S)-N-hydroxy-2-(isopropyl(methyl)amino)-3-(N-(4-(...)Show SMILES CC(C)N(C)[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C21H26F3N3O5S/c1-14(2)26(3)19(20(28)25-29)13-27(33(4,30)31)16-7-11-18(12-8-16)32-17-9-5-15(6-10-17)21(22,23)24/h5-12,14,19,29H,13H2,1-4H3,(H,25,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP9 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230506

((S)-N-hydroxy-2-(isopropylamino)-3-(N-(4-(4-(trifl...)Show SMILES CC(C)N[C@@H](CN(c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1)S(C)(=O)=O)C(=O)NO Show InChI InChI=1S/C20H24F3N3O5S/c1-13(2)24-18(19(27)25-28)12-26(32(3,29)30)15-6-10-17(11-7-15)31-16-8-4-14(5-9-16)20(21,22)23/h4-11,13,18,24,28H,12H2,1-3H3,(H,25,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230514

((S)-N-hydroxy-2-morpholino-3-(N-(4-(4-(trifluorome...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCOCC1)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C21H24F3N3O6S/c1-34(30,31)27(14-19(20(28)25-29)26-10-12-32-13-11-26)16-4-8-18(9-5-16)33-17-6-2-15(3-7-17)21(22,23)24/h2-9,19,29H,10-14H2,1H3,(H,25,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230505

((S)-N-hydroxy-2-(pyridin-4-ylmethylamino)-3-(N-(4-...)Show SMILES Cc1ccc(Oc2ccc(cc2)N(C[C@H](NCc2ccncc2)C(=O)NO)S(C)(=O)=O)cc1 Show InChI InChI=1S/C23H26N4O5S/c1-17-3-7-20(8-4-17)32-21-9-5-19(6-10-21)27(33(2,30)31)16-22(23(28)26-29)25-15-18-11-13-24-14-12-18/h3-14,22,25,29H,15-16H2,1-2H3,(H,26,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50230498

((S)-N-hydroxy-2-(4-(methylsulfonyl)piperazin-1-yl)...)Show SMILES CS(=O)(=O)N(C[C@H](N1CCN(CC1)S(C)(=O)=O)C(=O)NO)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H27F3N4O7S2/c1-37(32,33)28-13-11-27(12-14-28)20(21(30)26-31)15-29(38(2,34)35)17-5-9-19(10-6-17)36-18-7-3-16(4-8-18)22(23,24)25/h3-10,20,31H,11-15H2,1-2H3,(H,26,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
Bioorg Med Chem Lett 18: 1140-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.129
BindingDB Entry DOI: 10.7270/Q2BP02JM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data