 Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50046586
Found 27 hits Enz. Inhib. hit(s) with all data for entry = 50046586 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120437
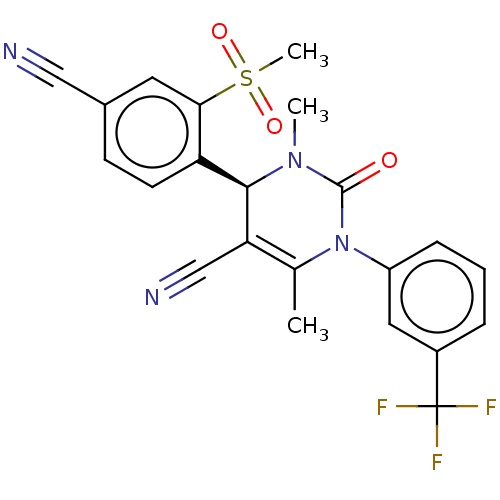
(CHEMBL3617973)Show SMILES CN1[C@@H](C(C#N)=C(C)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,t:5| Show InChI InChI=1S/C22H17F3N4O3S/c1-13-18(12-27)20(17-8-7-14(11-26)9-19(17)33(3,31)32)28(2)21(30)29(13)16-6-4-5-15(10-16)22(23,24)25/h4-10,20H,1-3H3/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118028
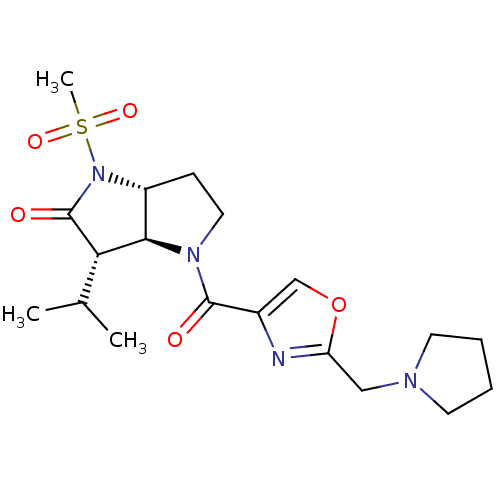
(3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2coc(CN3CCCC3)n2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H28N4O5S/c1-12(2)16-17-14(23(19(16)25)29(3,26)27)6-9-22(17)18(24)13-11-28-15(20-13)10-21-7-4-5-8-21/h11-12,14,16-17H,4-10H2,1-3H3/t14-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50035500
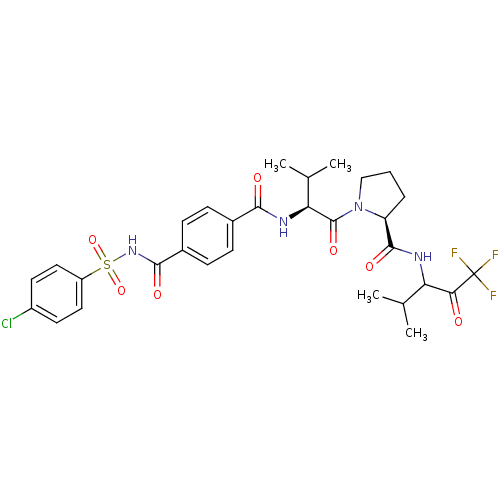
((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfonylaminocarb...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34ClF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50095522
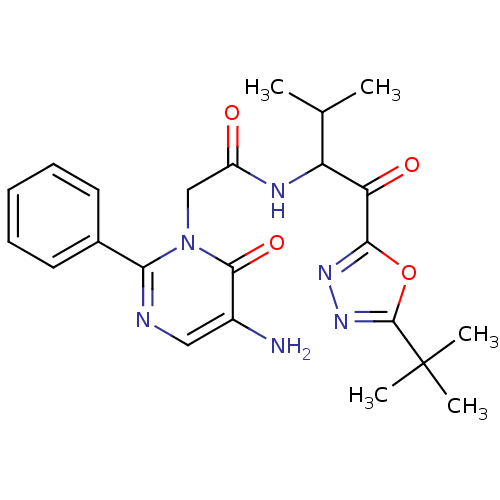
(2-(5-Amino-6-oxo-2-phenyl-6H-pyrimidin-1-yl)-N-[1-...)Show SMILES CC(C)C(NC(=O)Cn1c(ncc(N)c1=O)-c1ccccc1)C(=O)c1nnc(o1)C(C)(C)C Show InChI InChI=1S/C23H28N6O4/c1-13(2)17(18(31)20-27-28-22(33-20)23(3,4)5)26-16(30)12-29-19(14-9-7-6-8-10-14)25-11-15(24)21(29)32/h6-11,13,17H,12,24H2,1-5H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120430
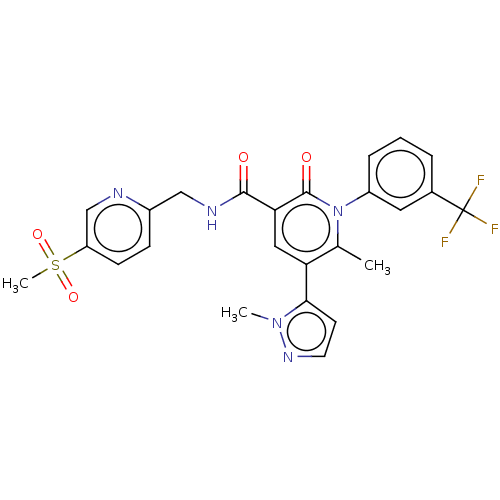
(CHEMBL3617964)Show SMILES Cc1c(cc(C(=O)NCc2ccc(cn2)S(C)(=O)=O)c(=O)n1-c1cccc(c1)C(F)(F)F)-c1ccnn1C Show InChI InChI=1S/C25H22F3N5O4S/c1-15-20(22-9-10-31-32(22)2)12-21(23(34)30-13-17-7-8-19(14-29-17)38(3,36)37)24(35)33(15)18-6-4-5-16(11-18)25(26,27)28/h4-12,14H,13H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeO-Suc-Ala-Ala-Pro-Val 7-amido-4-methylcoumarin as substrate preincubated for 15 mins followed by subs... |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120426
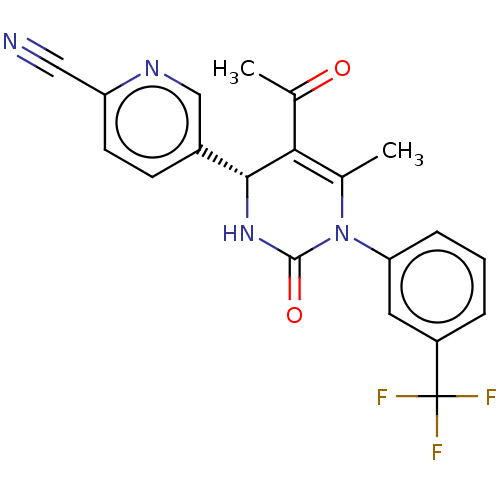
(CHEMBL3617968)Show SMILES CC(=O)C1=C(C)N(C(=O)N[C@@H]1c1ccc(nc1)C#N)c1cccc(c1)C(F)(F)F |r,c:3| Show InChI InChI=1S/C20H15F3N4O2/c1-11-17(12(2)28)18(13-6-7-15(9-24)25-10-13)26-19(29)27(11)16-5-3-4-14(8-16)20(21,22)23/h3-8,10,18H,1-2H3,(H,26,29)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120433
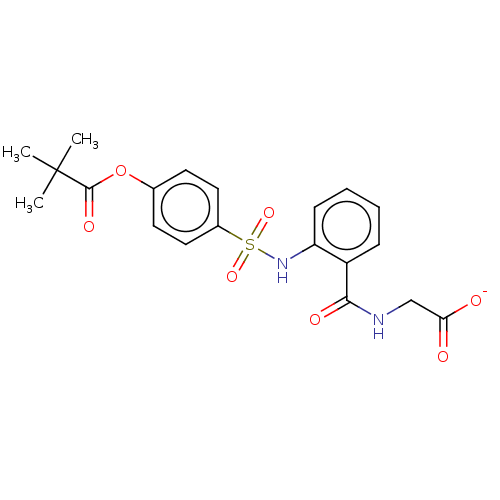
(ONO-5046.NA | Sivelestat Sodium)Show SMILES [Na+].CC(C)(C)C(=O)Oc1ccc(cc1)S(=O)(=O)Nc1ccccc1C(=O)NCC([O-])=O Show InChI InChI=1S/C20H22N2O7S.Na/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24;/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using suc-Ala-Pro-Ala-pNA as substrate after 30 mins by spectrophotometric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50349435
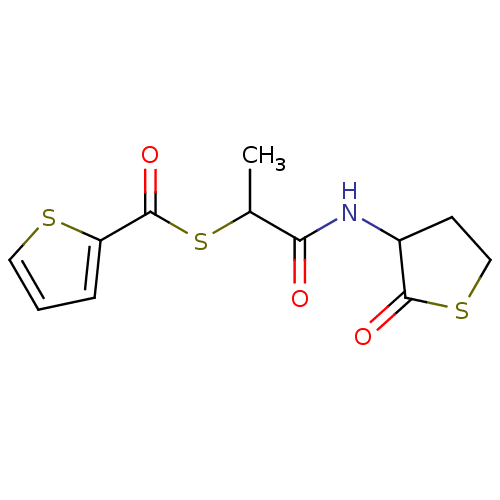
(CHEMBL1808549)Show InChI InChI=1S/C12H13NO3S3/c1-7(19-12(16)9-3-2-5-17-9)10(14)13-8-4-6-18-11(8)15/h2-3,5,7-8H,4,6H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120428
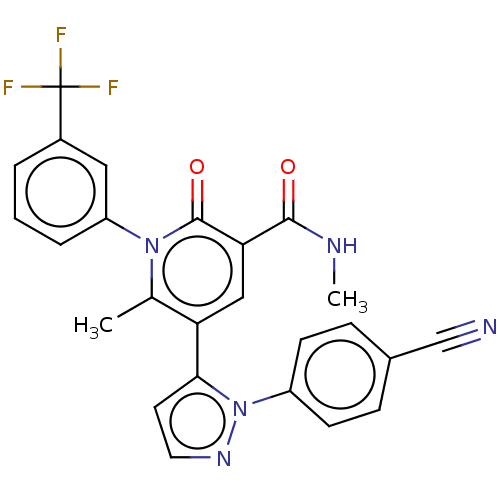
(CHEMBL3617966)Show SMILES CNC(=O)c1cc(-c2ccnn2-c2ccc(cc2)C#N)c(C)n(-c2cccc(c2)C(F)(F)F)c1=O Show InChI InChI=1S/C25H18F3N5O2/c1-15-20(22-10-11-31-33(22)18-8-6-16(14-29)7-9-18)13-21(23(34)30-2)24(35)32(15)19-5-3-4-17(12-19)25(26,27)28/h3-13H,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120436
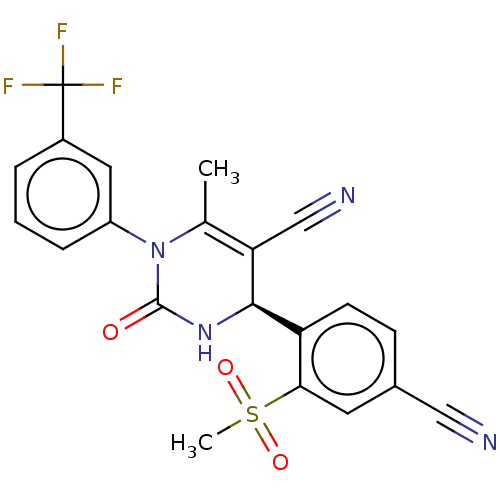
(CHEMBL3617972)Show SMILES CC1=C(C#N)[C@H](NC(=O)N1c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,c:1| Show InChI InChI=1S/C21H15F3N4O3S/c1-12-17(11-26)19(16-7-6-13(10-25)8-18(16)32(2,30)31)27-20(29)28(12)15-5-3-4-14(9-15)21(22,23)24/h3-9,19H,1-2H3,(H,27,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeOSuc-AAPV-AMC as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120429
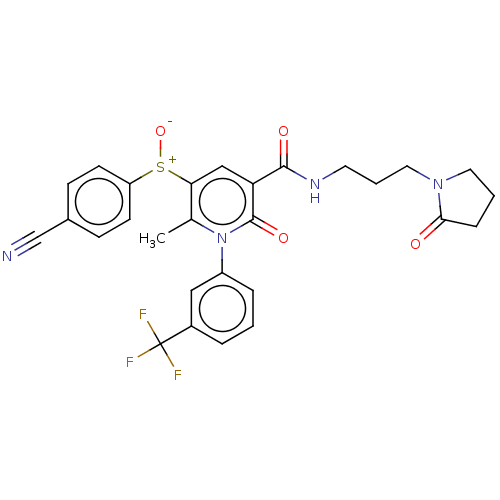
(CHEMBL3617965)Show SMILES Cc1c(cc(C(=O)NCCCN2CCCC2=O)c(=O)n1-c1cccc(c1)C(F)(F)F)[S+]([O-])c1ccc(cc1)C#N Show InChI InChI=1S/C28H25F3N4O4S/c1-18-24(40(39)22-10-8-19(17-32)9-11-22)16-23(26(37)33-12-4-14-34-13-3-7-25(34)36)27(38)35(18)21-6-2-5-20(15-21)28(29,30)31/h2,5-6,8-11,15-16H,3-4,7,12-14H2,1H3,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120424
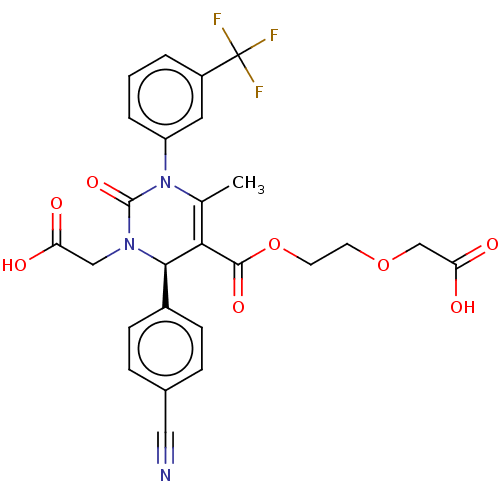
(CHEMBL3617970)Show SMILES CC1=C([C@H](N(CC(O)=O)C(=O)N1c1cccc(c1)C(F)(F)F)c1ccc(cc1)C#N)C(=O)OCCOCC(O)=O |r,t:1| Show InChI InChI=1S/C26H22F3N3O8/c1-15-22(24(37)40-10-9-39-14-21(35)36)23(17-7-5-16(12-30)6-8-17)31(13-20(33)34)25(38)32(15)19-4-2-3-18(11-19)26(27,28)29/h2-8,11,23H,9-10,13-14H2,1H3,(H,33,34)(H,35,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120440
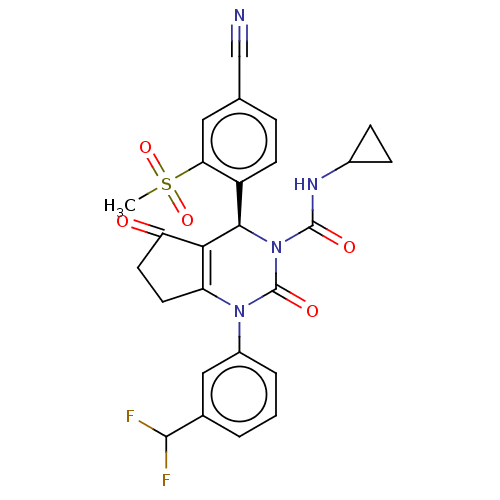
(CHEMBL3617976)Show SMILES CS(=O)(=O)c1cc(ccc1[C@H]1N(C(=O)NC2CC2)C(=O)N(C2=C1C(=O)CC2)c1cccc(c1)C(F)F)C#N |r,c:23| Show InChI InChI=1S/C26H22F2N4O5S/c1-38(36,37)21-11-14(13-29)5-8-18(21)23-22-19(9-10-20(22)33)31(17-4-2-3-15(12-17)24(27)28)26(35)32(23)25(34)30-16-6-7-16/h2-5,8,11-12,16,23-24H,6-7,9-10H2,1H3,(H,30,34)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human plasma using MeOSuc-Ala-Ala-Pro-Val-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120440

(CHEMBL3617976)Show SMILES CS(=O)(=O)c1cc(ccc1[C@H]1N(C(=O)NC2CC2)C(=O)N(C2=C1C(=O)CC2)c1cccc(c1)C(F)F)C#N |r,c:23| Show InChI InChI=1S/C26H22F2N4O5S/c1-38(36,37)21-11-14(13-29)5-8-18(21)23-22-19(9-10-20(22)33)31(17-4-2-3-15(12-17)24(27)28)26(35)32(23)25(34)30-16-6-7-16/h2-5,8,11-12,16,23-24H,6-7,9-10H2,1H3,(H,30,34)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeO-Succ-Ala-Ala-Pro-Val-AMC as substrate after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120439
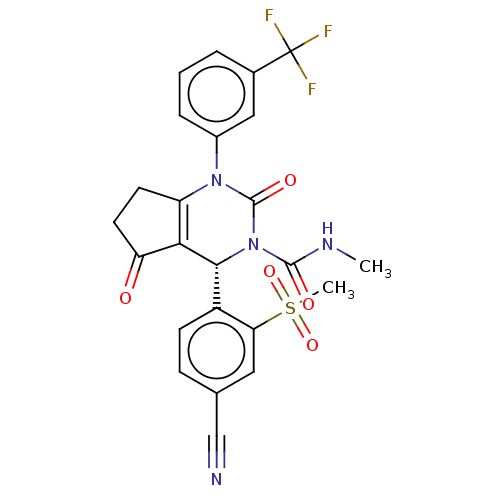
(CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1)Show SMILES CNC(=O)N1[C@@H](C2=C(CCC2=O)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,t:6| Show InChI InChI=1S/C24H19F3N4O5S/c1-29-22(33)31-21(16-7-6-13(12-28)10-19(16)37(2,35)36)20-17(8-9-18(20)32)30(23(31)34)15-5-3-4-14(11-15)24(25,26)27/h3-7,10-11,21H,8-9H2,1-2H3,(H,29,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human plasma using MeOSuc-Ala-Ala-Pro-Val-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120439

(CHEMBL3617975 | US9290459, 47.1 | US9670166, 47.1)Show SMILES CNC(=O)N1[C@@H](C2=C(CCC2=O)N(C1=O)c1cccc(c1)C(F)(F)F)c1ccc(cc1S(C)(=O)=O)C#N |r,t:6| Show InChI InChI=1S/C24H19F3N4O5S/c1-29-22(33)31-21(16-7-6-13(12-28)10-19(16)37(2,35)36)20-17(8-9-18(20)32)30(23(31)34)15-5-3-4-14(11-15)24(25,26)27/h3-7,10-11,21H,8-9H2,1-2H3,(H,29,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeO-Succ-Ala-Ala-Pro-Val-AMC as substrate after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120438
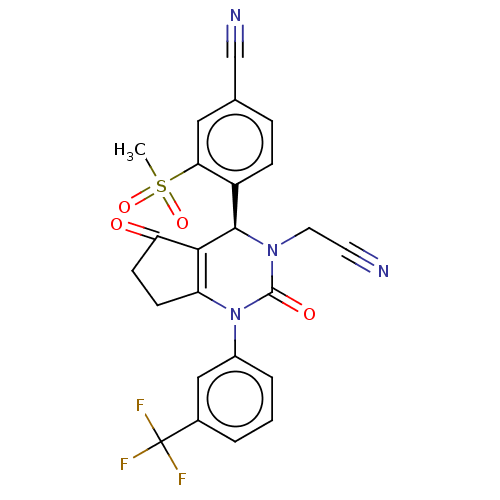
(CHEMBL3617974 | US9290459, 23 | US9670166, 23)Show SMILES CS(=O)(=O)c1cc(ccc1[C@H]1N(CC#N)C(=O)N(C2=C1C(=O)CC2)c1cccc(c1)C(F)(F)F)C#N |r,c:19| Show InChI InChI=1S/C24H17F3N4O4S/c1-36(34,35)20-11-14(13-29)5-6-17(20)22-21-18(7-8-19(21)32)31(23(33)30(22)10-9-28)16-4-2-3-15(12-16)24(25,26)27/h2-6,11-12,22H,7-8,10H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human plasma using MeOSuc-Ala-Ala-Pro-Val-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120438

(CHEMBL3617974 | US9290459, 23 | US9670166, 23)Show SMILES CS(=O)(=O)c1cc(ccc1[C@H]1N(CC#N)C(=O)N(C2=C1C(=O)CC2)c1cccc(c1)C(F)(F)F)C#N |r,c:19| Show InChI InChI=1S/C24H17F3N4O4S/c1-36(34,35)20-11-14(13-29)5-6-17(20)22-21-18(7-8-19(21)32)31(23(33)30(22)10-9-28)16-4-2-3-15(12-16)24(25,26)27/h2-6,11-12,22H,7-8,10H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeO-Succ-Ala-Ala-Pro-Val-AMC as substrate after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120425
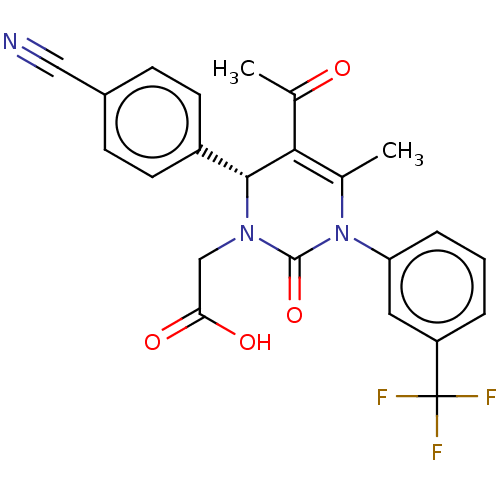
(CHEMBL3617969)Show SMILES CC(=O)C1=C(C)N(C(=O)N(CC(O)=O)[C@@H]1c1ccc(cc1)C#N)c1cccc(c1)C(F)(F)F |r,c:3| Show InChI InChI=1S/C23H18F3N3O4/c1-13-20(14(2)30)21(16-8-6-15(11-27)7-9-16)28(12-19(31)32)22(33)29(13)18-5-3-4-17(10-18)23(24,25)26/h3-10,21H,12H2,1-2H3,(H,31,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120434
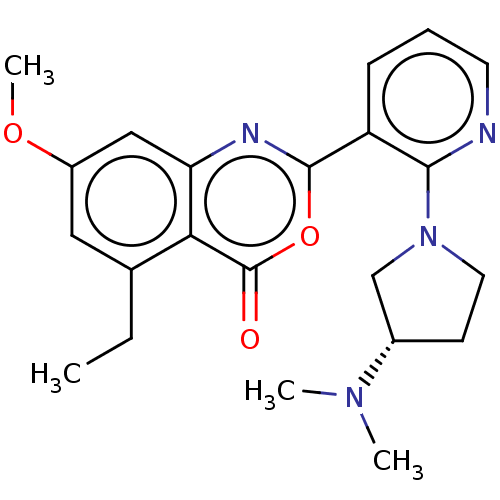
(CHEMBL3617961)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CC[C@@H](C1)N(C)C |r| Show InChI InChI=1S/C22H26N4O3/c1-5-14-11-16(28-4)12-18-19(14)22(27)29-21(24-18)17-7-6-9-23-20(17)26-10-8-15(13-26)25(2)3/h6-7,9,11-12,15H,5,8,10,13H2,1-4H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120431

(CHEMBL3617963)Show SMILES Cc1noc(C)c1-c1cc(C(=O)NCc2ccc(cc2)S(C)(=O)=O)c(=O)n(-c2cccc(Cl)c2)c1C |(4.3,-4.31,;4.06,-5.52,;5.09,-6.66,;4.33,-7.99,;2.82,-7.68,;1.9,-8.5,;2.67,-6.16,;1.34,-5.39,;0,-6.16,;-1.33,-5.39,;-2.66,-6.17,;-3.73,-5.56,;-2.66,-7.71,;-3.99,-8.48,;-3.98,-10.03,;-5.31,-10.8,;-5.31,-12.34,;-3.97,-13.11,;-2.64,-12.33,;-2.65,-10.79,;-3.96,-14.65,;-3.95,-15.88,;-5.02,-15.27,;-2.89,-15.26,;-1.33,-3.85,;-2.4,-3.24,;,-3.08,;,-1.54,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.4,1.39,;-1.33,-.77,;1.33,-3.85,;2.4,-3.23,)| Show InChI InChI=1S/C26H24ClN3O5S/c1-15-24(17(3)35-29-15)22-13-23(26(32)30(16(22)2)20-7-5-6-19(27)12-20)25(31)28-14-18-8-10-21(11-9-18)36(4,33)34/h5-13H,14H2,1-4H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120454
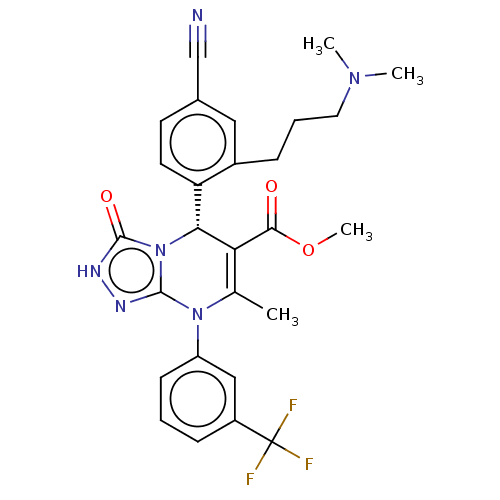
(CHEMBL3617977)Show SMILES COC(=O)C1=C(C)N(c2n[nH]c(=O)n2[C@@H]1c1ccc(cc1CCCN(C)C)C#N)c1cccc(c1)C(F)(F)F |r,c:4| Show InChI InChI=1S/C27H27F3N6O3/c1-16-22(24(37)39-4)23(21-11-10-17(15-31)13-18(21)7-6-12-34(2)3)36-25(32-33-26(36)38)35(16)20-9-5-8-19(14-20)27(28,29)30/h5,8-11,13-14,23H,6-7,12H2,1-4H3,(H,33,38)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase using MeO-Succ-Ala-Ala-Pro-Val-AMC as substrate after 30 mins by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120427

(CHEMBL3617967)Show SMILES CN(C)CC(=O)Nc1nc2c(c(C)c(cn2n1)-c1ccnn1-c1ccc(cc1)C#N)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C28H23F3N8O/c1-17-22(23-11-12-33-39(23)21-9-7-18(14-32)8-10-21)15-38-26(35-27(36-38)34-24(40)16-37(2)3)25(17)19-5-4-6-20(13-19)28(29,30)31/h4-13,15H,16H2,1-3H3,(H,34,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120423
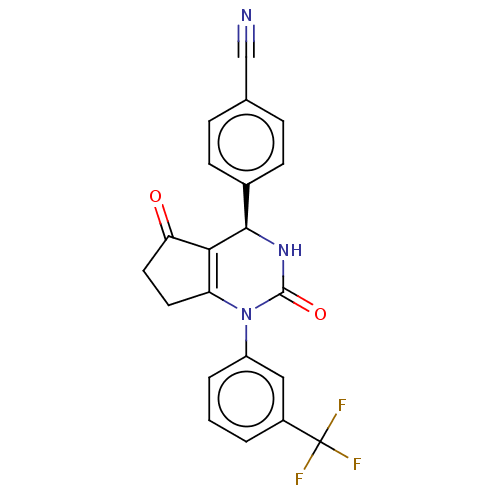
(CHEMBL3617971 | US9290459, 1A | US9670166, 1A)Show SMILES FC(F)(F)c1cccc(c1)N1C2=C([C@H](NC1=O)c1ccc(cc1)C#N)C(=O)CC2 |r,c:12| Show InChI InChI=1S/C21H14F3N3O2/c22-21(23,24)14-2-1-3-15(10-14)27-16-8-9-17(28)18(16)19(26-20(27)29)13-6-4-12(11-25)5-7-13/h1-7,10,19H,8-9H2,(H,26,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human plasma using MeOSuc-Ala-Ala-Pro-Val-AMC as substrate by fluorescence assay |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118029

(3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...)Show SMILES Cl.[H][C@@]12CCN(C(=O)\C=C\CN3CCCCC3)[C@@]1([H])[C@H](C(C)C)C(=O)N2S(C)(=O)=O |r| Show InChI InChI=1S/C19H31N3O4S/c1-14(2)17-18-15(22(19(17)24)27(3,25)26)9-13-21(18)16(23)8-7-12-20-10-5-4-6-11-20/h7-8,14-15,17-18H,4-6,9-13H2,1-3H3/b8-7+/t15-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of neutrophil elastase in human whole blood using MeO-Succ-Ala-Ala-Pro-Val-pNA as substrate after 30 mins by colorimetric analysis |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120423

(CHEMBL3617971 | US9290459, 1A | US9670166, 1A)Show SMILES FC(F)(F)c1cccc(c1)N1C2=C([C@H](NC1=O)c1ccc(cc1)C#N)C(=O)CC2 |r,c:12| Show InChI InChI=1S/C21H14F3N3O2/c22-21(23,24)14-2-1-3-15(10-14)27-16-8-9-17(28)18(16)19(26-20(27)29)13-6-4-12(11-25)5-7-13/h1-7,10,19H,8-9H2,(H,26,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50120432
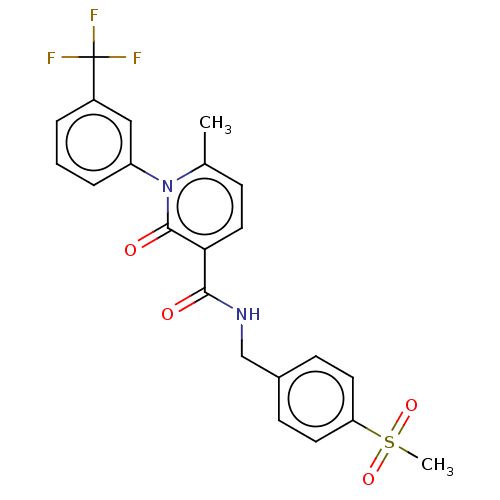
(CHEMBL3617962)Show SMILES Cc1ccc(C(=O)NCc2ccc(cc2)S(C)(=O)=O)c(=O)n1-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H19F3N2O4S/c1-14-6-11-19(20(28)26-13-15-7-9-18(10-8-15)32(2,30)31)21(29)27(14)17-5-3-4-16(12-17)22(23,24)25/h3-12H,13H2,1-2H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG
Curated by ChEMBL
| Assay Description
Inhibition of human neutrophil elastase |
Bioorg Med Chem Lett 25: 4370-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.049
BindingDB Entry DOI: 10.7270/Q29Z96PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data