 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50046606
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50046606 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50121212
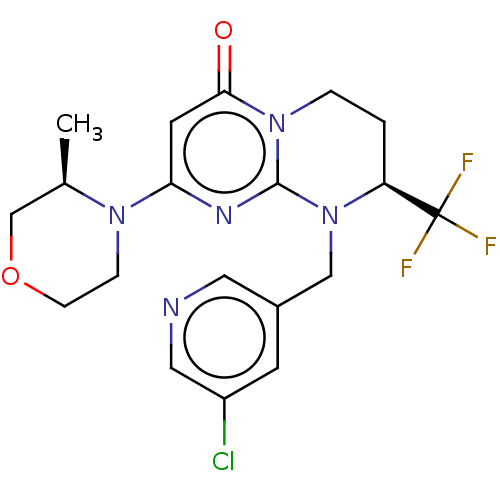
(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Vps34 by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHKKAc as substrate by fluorescence assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Endoplasmin
(Homo sapiens (Human)) | BDBM50087812
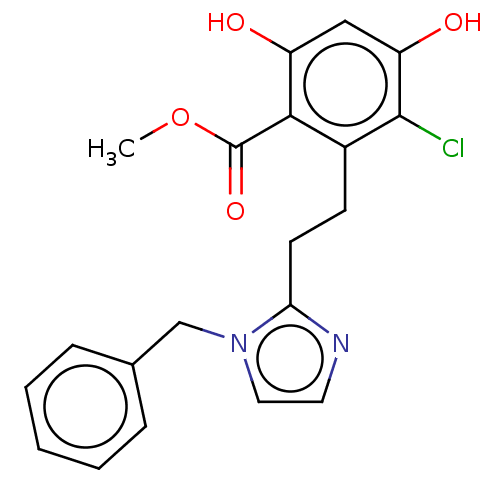
(CHEMBL3426787)Show SMILES COC(=O)c1c(O)cc(O)c(Cl)c1CCc1nccn1Cc1ccccc1 Show InChI InChI=1S/C20H19ClN2O4/c1-27-20(26)18-14(19(21)16(25)11-15(18)24)7-8-17-22-9-10-23(17)12-13-5-3-2-4-6-13/h2-6,9-11,24-25H,7-8,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GRP94 (unknown origin) expressed in HEK293 cells assessed as reduction of Toll-like receptor trafficking to cell membrane treated for 2... |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) after 20 mins by radiometric analysis in presence of [gamma33P]-ATP |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50121210
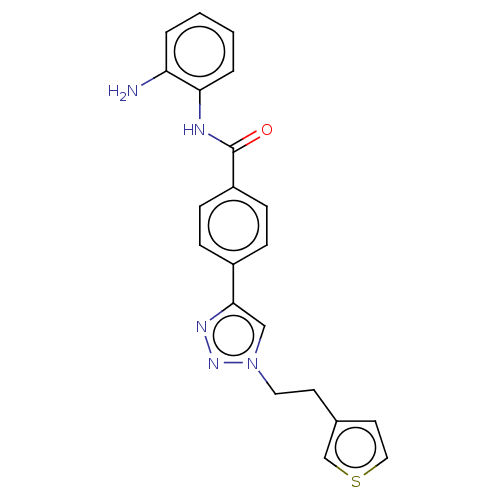
(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50121213

(CHEMBL3622371)Show SMILES O=C(NCc1ccccc1)[C@H](CCCCNC1CC1c1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H33N3O2/c33-28(24-16-8-3-9-17-24)32-26(29(34)31-21-22-12-4-1-5-13-22)18-10-11-19-30-27-20-25(27)23-14-6-2-7-15-23/h1-9,12-17,25-27,30H,10-11,18-21H2,(H,31,34)(H,32,33)/t25?,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50346862
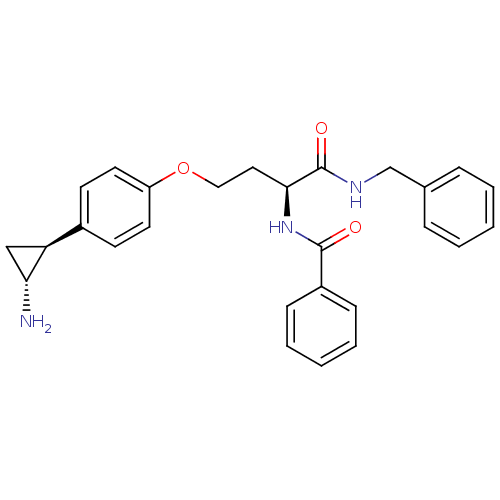
(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal hexahistidine-tagged human LSD1 (1 to 852 amino acid residues) expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptid... |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) after 20 mins by radiometric analysis in presence of [gamma33P]-ATP |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50445336
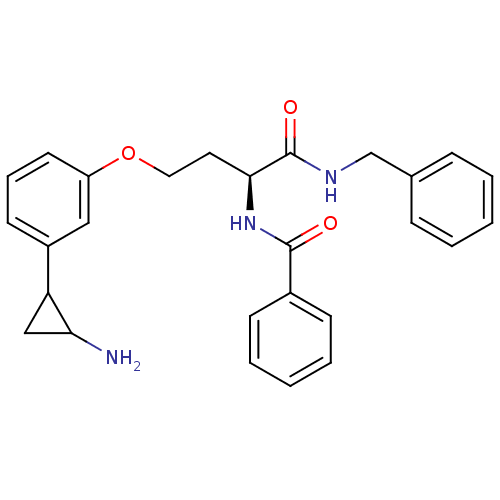
(CHEMBL1797639)Show SMILES NC1CC1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23?,24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal hexahistidine-tagged human LSD1 (1 to 852 amino acid residues) expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptid... |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) after 20 mins by radiometric analysis in presence of [gamma33P]-ATP |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50113851
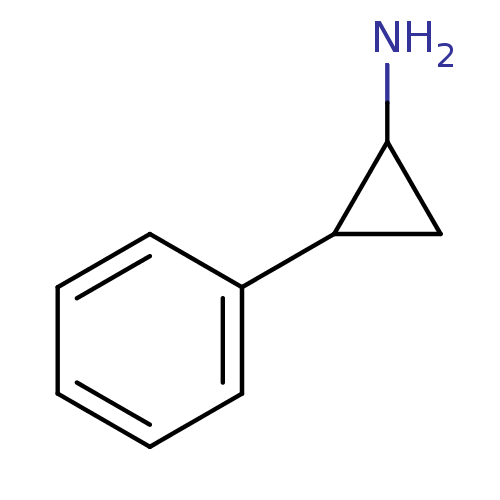
((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-B using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50121209

(CHEMBL3622376)Show InChI InChI=1S/C11H10N6O/c1-2-12-11-8(1)10(13-6-14-11)9-4-17(16-15-9)3-7-5-18-7/h1-2,4,6-7H,3,5H2,(H,12,13,14) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 (unknown origin) after 20 mins by radiometric analysis in presence of [gamma33P]-ATP |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50113851

((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-A using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50121212

(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kbeta by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50121212

(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kalpha by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50121212

(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kgamma by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50121212

(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3Kdelta by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50121212

(CHEMBL3622372)Show SMILES C[C@@H]1COCCN1c1cc(=O)n2CC[C@H](N(Cc3cncc(Cl)c3)c2n1)C(F)(F)F |r| Show InChI InChI=1S/C19H21ClF3N5O2/c1-12-11-30-5-4-26(12)16-7-17(29)27-3-2-15(19(21,22)23)28(18(27)25-16)10-13-6-14(20)9-24-8-13/h6-9,12,15H,2-5,10-11H2,1H3/t12-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human mTOR by TR-FRET analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50121213

(CHEMBL3622371)Show SMILES O=C(NCc1ccccc1)[C@H](CCCCNC1CC1c1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H33N3O2/c33-28(24-16-8-3-9-17-24)32-26(29(34)31-21-22-12-4-1-5-13-22)18-10-11-19-30-27-20-25(27)23-14-6-2-7-15-23/h1-9,12-17,25-27,30H,10-11,18-21H2,(H,31,34)(H,32,33)/t25?,26-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A (unknown origin) |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50113851

((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of LSD1 (unknown origin) |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50113851

((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal hexahistidine-tagged human LSD1 (1 to 852 amino acid residues) expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptid... |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50121211

(CHEMBL3622375)Show SMILES Nc1ccccc1NC(=O)c1ccc(s1)-c1cn(CCc2cccc(c2)[N+]([O-])=O)nn1 Show InChI InChI=1S/C21H18N6O3S/c22-16-6-1-2-7-17(16)23-21(28)20-9-8-19(31-20)18-13-26(25-24-18)11-10-14-4-3-5-15(12-14)27(29)30/h1-9,12-13H,10-11,22H2,(H,23,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50121210

(CHEMBL3622374)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)-c1cn(CCc2ccsc2)nn1 Show InChI InChI=1S/C21H19N5OS/c22-18-3-1-2-4-19(18)23-21(27)17-7-5-16(6-8-17)20-13-26(25-24-20)11-9-15-10-12-28-14-15/h1-8,10,12-14H,9,11,22H2,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa nuclear extracts by fluorometric assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50445336

(CHEMBL1797639)Show SMILES NC1CC1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23?,24?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-A using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50346862

(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-A using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50445336

(CHEMBL1797639)Show SMILES NC1CC1c1cccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)c1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)21-12-7-13-22(16-21)33-15-14-25(30-26(31)20-10-5-2-6-11-20)27(32)29-18-19-8-3-1-4-9-19/h1-13,16,23-25H,14-15,17-18,28H2,(H,29,32)(H,30,31)/t23?,24?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-B using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50346862

(CHEMBL1215658)Show SMILES N[C@@H]1C[C@H]1c1ccc(OCC[C@H](NC(=O)c2ccccc2)C(=O)NCc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H29N3O3/c28-24-17-23(24)20-11-13-22(14-12-20)33-16-15-25(30-26(31)21-9-5-2-6-10-21)27(32)29-18-19-7-3-1-4-8-19/h1-14,23-25H,15-18,28H2,(H,29,32)(H,30,31)/t23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human MAO-B using (4S)-4,5-dihydro-2-(6-hydroxybenzothiazolyl)-4-thiazolecarboxylic acid as substrate after 60 mins by MAO-Glo assay |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM188519
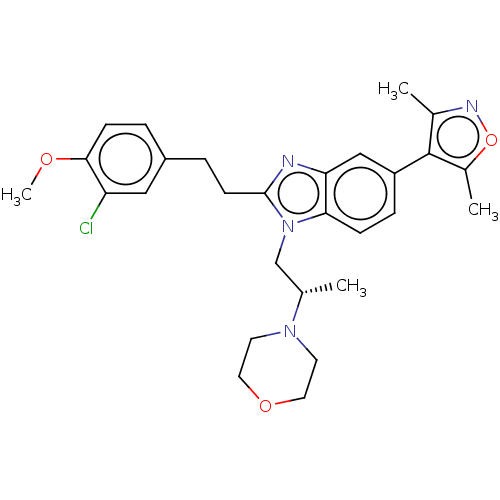
(SGC-CBP30)Show SMILES COc1ccc(CCc2nc3cc(ccc3n2C[C@H](C)N2CCOCC2)-c2c(C)noc2C)cc1Cl |r| Show InChI InChI=1S/C28H33ClN4O3/c1-18(32-11-13-35-14-12-32)17-33-25-8-7-22(28-19(2)31-36-20(28)3)16-24(25)30-27(33)10-6-21-5-9-26(34-4)23(29)15-21/h5,7-9,15-16,18H,6,10-14,17H2,1-4H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to human CBP by ITC analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM188519

(SGC-CBP30)Show SMILES COc1ccc(CCc2nc3cc(ccc3n2C[C@H](C)N2CCOCC2)-c2c(C)noc2C)cc1Cl |r| Show InChI InChI=1S/C28H33ClN4O3/c1-18(32-11-13-35-14-12-32)17-33-25-8-7-22(28-19(2)31-36-20(28)3)16-24(25)30-27(33)10-6-21-5-9-26(34-4)23(29)15-21/h5,7-9,15-16,18H,6,10-14,17H2,1-4H3/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to human p300 by ITC analysis |
J Med Chem 58: 7611-33 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00229
BindingDB Entry DOI: 10.7270/Q2QZ2CSD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data