 Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50026275
Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50026275 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
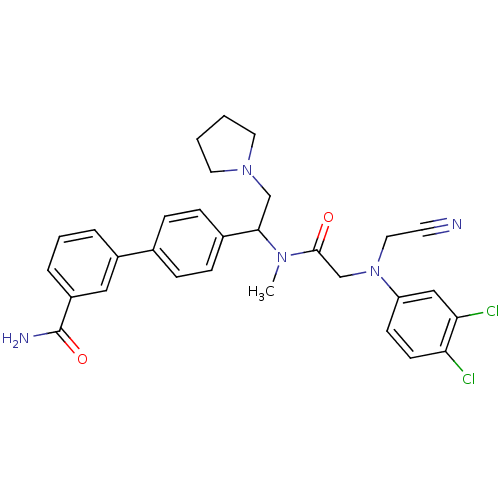
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244022
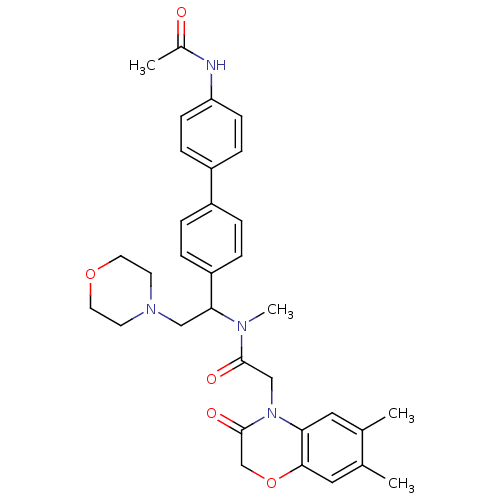
(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244019
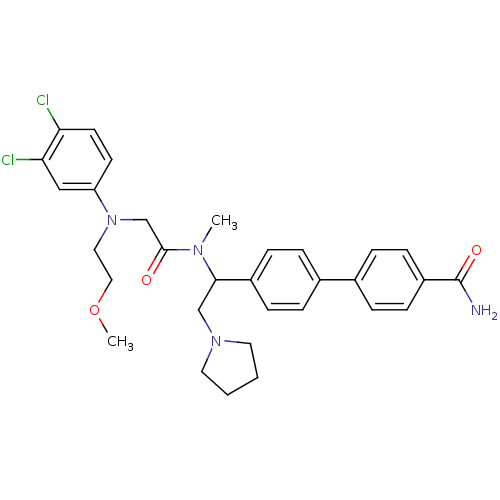
(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244021
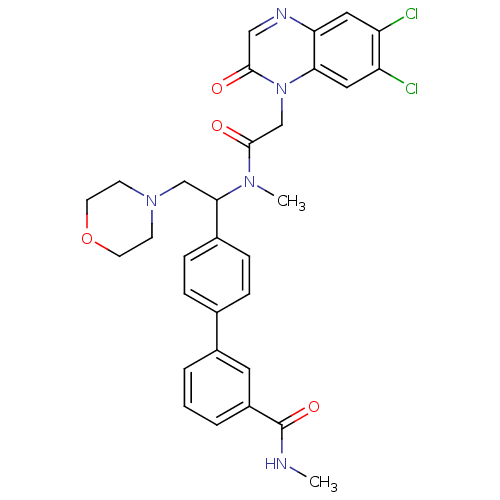
(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244065
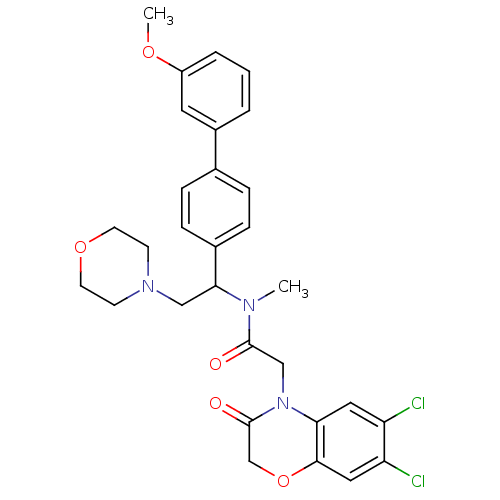
(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50239135
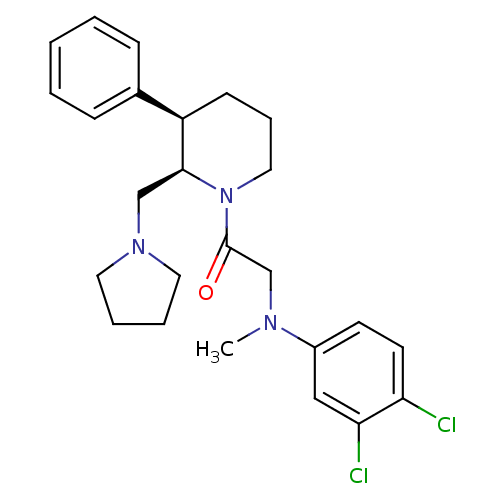
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2R,3R)-3...)Show SMILES CN(CC(=O)N1CCC[C@@H]([C@@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244018
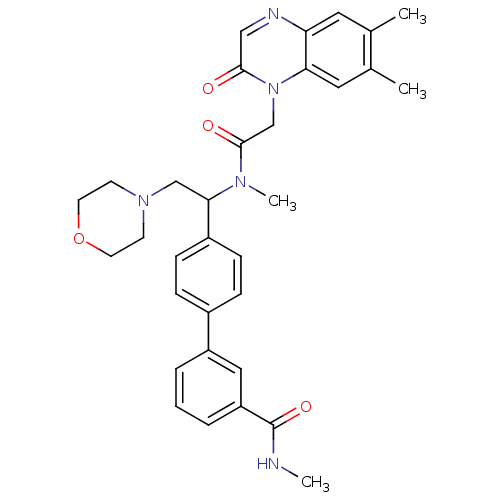
(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243970
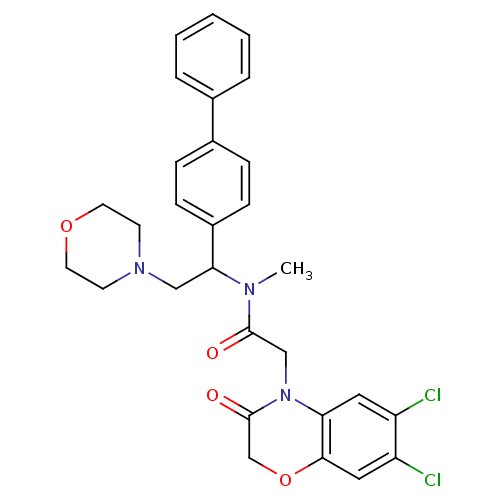
((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243919
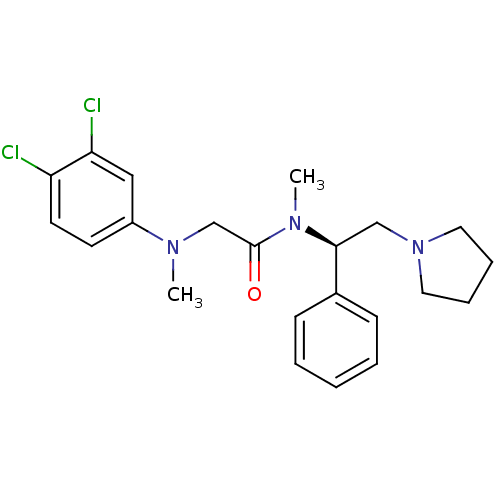
((R)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243868
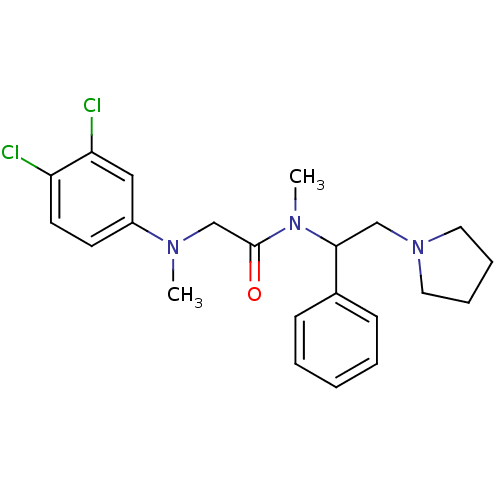
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243971
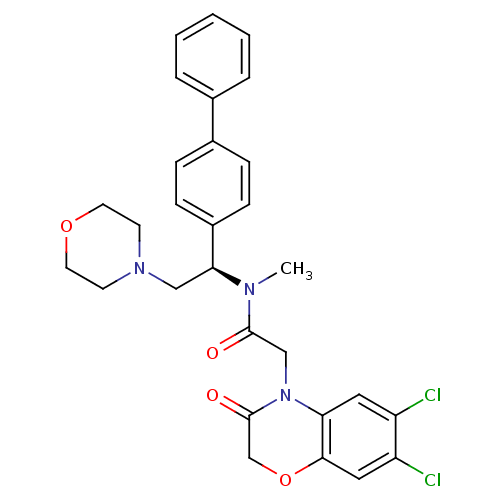
(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50240153
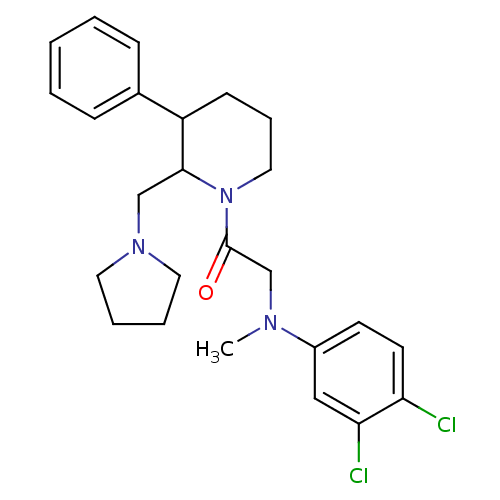
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243921
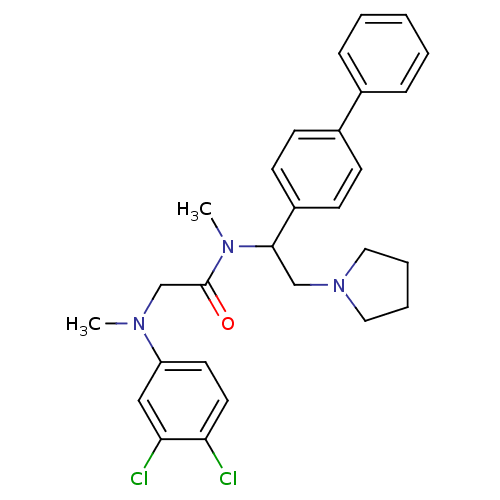
(CHEMBL488642 | N-(1-Biphenyl-4-yl-2-pyrrolidin-1-y...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O/c1-31(24-14-15-25(29)26(30)18-24)20-28(34)32(2)27(19-33-16-6-7-17-33)23-12-10-22(11-13-23)21-8-4-3-5-9-21/h3-5,8-15,18,27H,6-7,16-17,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243969
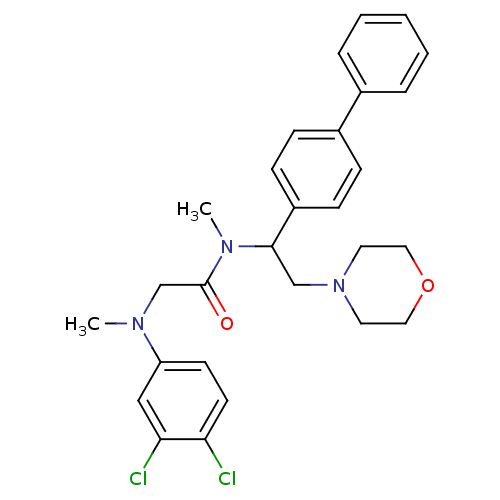
(CHEMBL452298 | N-(1-Biphenyl-4-yl-2-morpholin-4-yl...)Show SMILES CN(CC(=O)N(C)C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O2/c1-31(24-12-13-25(29)26(30)18-24)20-28(34)32(2)27(19-33-14-16-35-17-15-33)23-10-8-22(9-11-23)21-6-4-3-5-7-21/h3-13,18,27H,14-17,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244017
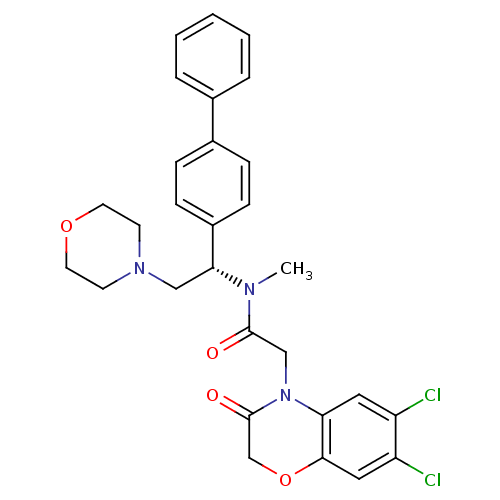
(CHEMBL452808 | N-((S)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50241424
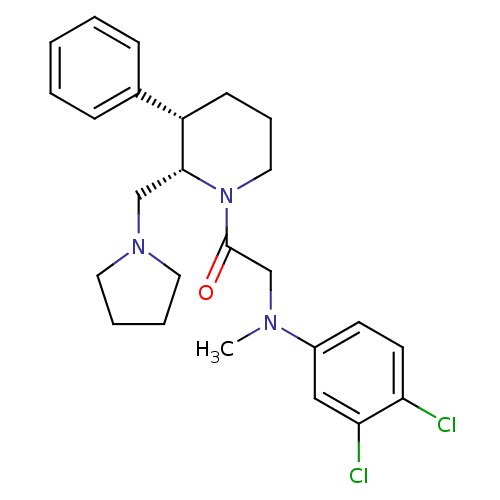
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2S,3S)-3...)Show SMILES CN(CC(=O)N1CCC[C@H]([C@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243920
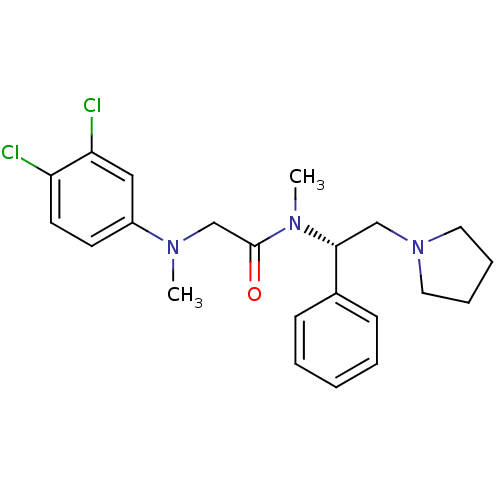
((S)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50007344
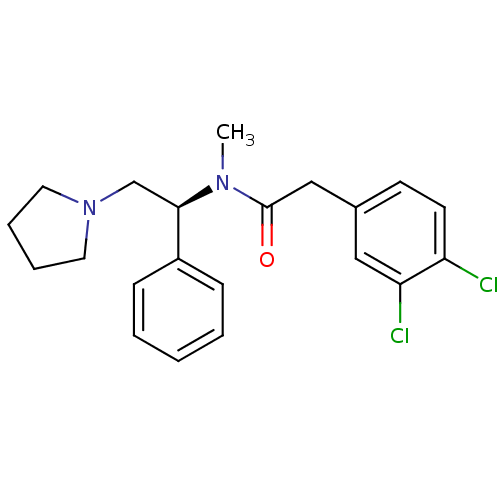
((S)-2-(3,4-Dichloro-phenyl)-N-methyl-N-(1-phenyl-2...)Show SMILES CN([C@H](CN1CCCC1)c1ccccc1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H24Cl2N2O/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243868

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50240153

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244065

(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50240153

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243969

(CHEMBL452298 | N-(1-Biphenyl-4-yl-2-morpholin-4-yl...)Show SMILES CN(CC(=O)N(C)C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O2/c1-31(24-12-13-25(29)26(30)18-24)20-28(34)32(2)27(19-33-14-16-35-17-15-33)23-10-8-22(9-11-23)21-6-4-3-5-7-21/h3-13,18,27H,14-17,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244020

(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244019

(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243868

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244020

(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244017

(CHEMBL452808 | N-((S)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243970

((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243921

(CHEMBL488642 | N-(1-Biphenyl-4-yl-2-pyrrolidin-1-y...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O/c1-31(24-14-15-25(29)26(30)18-24)20-28(34)32(2)27(19-33-16-6-7-17-33)23-12-10-22(11-13-23)21-8-4-3-5-9-21/h3-5,8-15,18,27H,6-7,16-17,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244021

(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243921

(CHEMBL488642 | N-(1-Biphenyl-4-yl-2-pyrrolidin-1-y...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O/c1-31(24-14-15-25(29)26(30)18-24)20-28(34)32(2)27(19-33-16-6-7-17-33)23-12-10-22(11-13-23)21-8-4-3-5-9-21/h3-5,8-15,18,27H,6-7,16-17,19-20H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244019

(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244022

(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50244018

(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50243971

(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243969

(CHEMBL452298 | N-(1-Biphenyl-4-yl-2-morpholin-4-yl...)Show SMILES CN(CC(=O)N(C)C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O2/c1-31(24-12-13-25(29)26(30)18-24)20-28(34)32(2)27(19-33-14-16-35-17-15-33)23-10-8-22(9-11-23)21-6-4-3-5-7-21/h3-13,18,27H,14-17,19-20H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244065

(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244022

(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244021

(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243971

(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244017

(CHEMBL452808 | N-((S)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50244018

(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50243970

((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244018

(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244019

(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244020

(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244021

(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244022

(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50244065

(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at kappa opioid receptor (unknown origin) |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data