 Found 78 hits Enz. Inhib. hit(s) with all data for entry = 50026446
Found 78 hits Enz. Inhib. hit(s) with all data for entry = 50026446 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252278
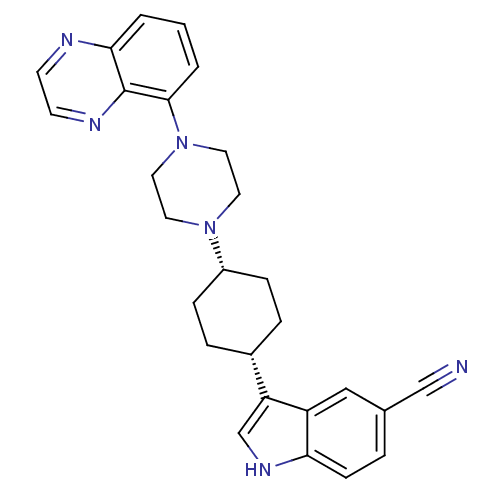
(3-[(1,4-cis)-4-(4-Quinoxalin-5-yl-piperazin-1-yl)-...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4nccnc34)c2c1 |r,wU:9.8,12.15,(-5.41,-44.52,;-5,-46.02,;-4.7,-47.53,;-3.23,-48.02,;-2.93,-49.53,;-4.08,-50.54,;-4.1,-52.08,;-5.57,-52.54,;-6.46,-51.29,;-8,-51.27,;-8.76,-49.93,;-10.3,-49.91,;-11.07,-51.24,;-10.32,-52.58,;-8.78,-52.6,;-12.62,-51.23,;-13.4,-52.56,;-14.94,-52.55,;-15.69,-51.21,;-14.91,-49.88,;-13.38,-49.89,;-17.23,-51.21,;-18,-52.54,;-19.55,-52.53,;-20.32,-51.19,;-19.52,-49.86,;-20.29,-48.54,;-19.52,-47.21,;-17.98,-47.22,;-17.23,-48.55,;-17.99,-49.87,;-5.54,-50.04,;-5.84,-48.54,)| Show InChI InChI=1S/C27H28N6/c28-17-19-4-9-24-22(16-19)23(18-31-24)20-5-7-21(8-6-20)32-12-14-33(15-13-32)26-3-1-2-25-27(26)30-11-10-29-25/h1-4,9-11,16,18,20-21,31H,5-8,12-15H2/t20-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252221

(3-[(1,4-cis)-4-(4-Quinolin-8-yl-piperazin-1-yl)-cy...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:9.8,12.15,(14.01,-32.59,;14.42,-34.09,;14.73,-35.6,;16.19,-36.09,;16.49,-37.6,;15.34,-38.61,;15.33,-40.15,;13.85,-40.61,;12.96,-39.36,;11.42,-39.34,;10.66,-38,;9.12,-37.98,;8.35,-39.31,;9.1,-40.66,;10.64,-40.67,;6.81,-39.3,;6.03,-40.63,;4.49,-40.62,;3.73,-39.29,;4.51,-37.96,;6.04,-37.96,;2.19,-39.28,;1.42,-40.61,;-.12,-40.61,;-.89,-39.26,;-.11,-37.93,;-.87,-36.61,;-.1,-35.28,;1.44,-35.29,;2.2,-36.62,;1.43,-37.95,;13.88,-38.12,;13.58,-36.61,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-26-24(17-20)25(19-31-26)21-7-9-23(10-8-21)32-13-15-33(16-14-32)27-5-1-3-22-4-2-12-30-28(22)27/h1-6,11-12,17,19,21,23,31H,7-10,13-16H2/t21-,23+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252591

(3-{4-[(1,4-cis)-4-(1H-Indol-4-yl)-pipera-zinyl-1-y...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:12.15,9.8,(31.78,-11.65,;32.19,-10.16,;32.53,-8.66,;33.99,-8.2,;34.33,-6.71,;33.2,-5.67,;33.22,-4.13,;31.76,-3.64,;30.84,-4.88,;29.31,-4.86,;28.55,-3.52,;27.01,-3.5,;26.23,-4.83,;26.99,-6.18,;28.52,-6.19,;24.7,-4.82,;23.92,-6.15,;22.38,-6.14,;21.62,-4.81,;22.4,-3.48,;23.93,-3.48,;20.08,-4.8,;19.32,-3.46,;17.78,-3.45,;17,-4.78,;17.77,-6.13,;17.29,-7.6,;18.54,-8.51,;19.79,-7.6,;19.31,-6.13,;31.74,-6.12,;31.4,-7.62,)| Show InChI InChI=1S/C27H29N5/c28-17-19-4-9-26-23(16-19)24(18-30-26)20-5-7-21(8-6-20)31-12-14-32(15-13-31)27-3-1-2-25-22(27)10-11-29-25/h1-4,9-11,16,18,20-21,29-30H,5-8,12-15H2/t20-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252084
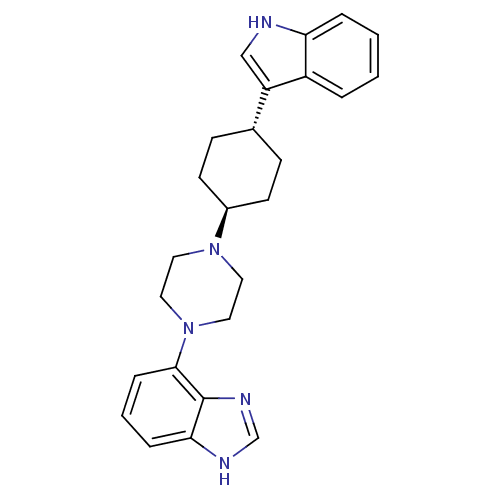
(4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin...)Show SMILES C1C[C@@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]cnc12)c1c[nH]c2ccccc12 |r,wU:2.24,wD:5.6,(32.34,.92,;33.88,.91,;34.67,2.24,;33.9,3.58,;32.36,3.6,;31.58,2.27,;30.04,2.28,;29.26,.94,;27.72,.96,;26.96,2.29,;27.74,3.63,;29.28,3.62,;25.42,2.3,;24.65,.97,;23.1,.97,;22.33,2.32,;23.11,3.65,;22.65,5.12,;23.9,6.02,;25.14,5.11,;24.66,3.64,;36.2,2.22,;37.09,.98,;38.55,1.44,;38.56,2.97,;39.71,3.97,;39.41,5.48,;37.95,5.96,;36.81,4.95,;37.11,3.46,)| Show InChI InChI=1S/C25H29N5/c1-2-5-22-20(4-1)21(16-26-22)18-8-10-19(11-9-18)29-12-14-30(15-13-29)24-7-3-6-23-25(24)28-17-27-23/h1-7,16-19,26H,8-15H2,(H,27,28)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252222
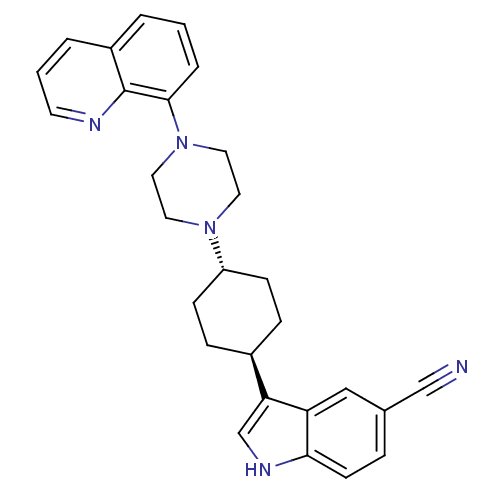
(3-[(1,4-trans)-4-(4-Quinolin-8-yl-piperazin-1-yl)-...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:12.15,wD:9.8,(34.65,-31.84,;35.06,-33.34,;35.37,-34.85,;36.83,-35.34,;37.13,-36.85,;35.98,-37.86,;35.96,-39.4,;34.49,-39.86,;33.6,-38.61,;32.06,-38.59,;31.3,-37.25,;29.76,-37.23,;28.98,-38.56,;29.74,-39.91,;31.27,-39.92,;27.44,-38.55,;26.67,-39.88,;25.13,-39.87,;24.37,-38.54,;25.14,-37.21,;26.68,-37.21,;22.83,-38.53,;22.06,-39.86,;20.52,-39.86,;19.75,-38.51,;20.53,-37.18,;19.77,-35.86,;20.54,-34.53,;22.08,-34.54,;22.84,-35.87,;22.06,-37.2,;34.52,-37.37,;34.22,-35.86,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-26-24(17-20)25(19-31-26)21-7-9-23(10-8-21)32-13-15-33(16-14-32)27-5-1-3-22-4-2-12-30-28(22)27/h1-6,11-12,17,19,21,23,31H,7-10,13-16H2/t21-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252026
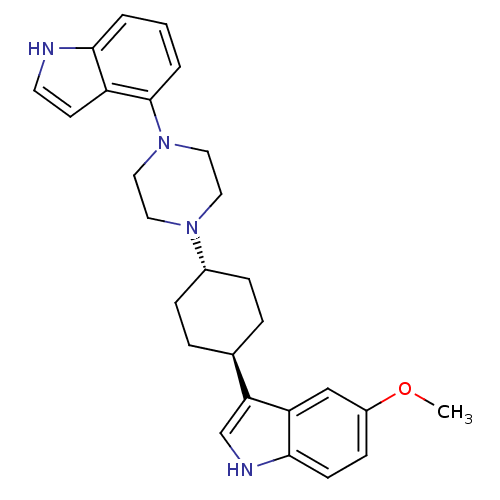
(5-Methoxy-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazi...)Show SMILES COc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:9.8,wD:12.15,(13.16,-32.09,;14.63,-31.63,;14.97,-30.13,;16.44,-29.67,;16.77,-28.18,;15.65,-27.14,;15.67,-25.6,;14.21,-25.11,;13.29,-26.35,;11.75,-26.33,;10.97,-27.66,;9.43,-27.64,;8.68,-26.3,;9.45,-24.97,;11,-24.99,;7.14,-26.29,;6.36,-27.62,;4.82,-27.61,;4.06,-26.28,;4.84,-24.95,;6.38,-24.95,;2.53,-26.27,;1.77,-24.93,;.23,-24.92,;-.55,-26.25,;.21,-27.6,;-.27,-29.07,;.99,-29.98,;2.23,-29.07,;1.75,-27.6,;14.18,-27.59,;13.85,-29.09,)| Show InChI InChI=1S/C27H32N4O/c1-32-21-9-10-26-23(17-21)24(18-29-26)19-5-7-20(8-6-19)30-13-15-31(16-14-30)27-4-2-3-25-22(27)11-12-28-25/h2-4,9-12,17-20,28-29H,5-8,13-16H2,1H3/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252129
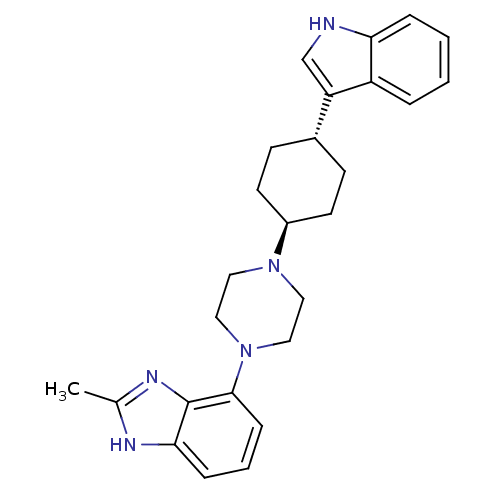
(4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]piperazin-...)Show SMILES Cc1nc2c(cccc2[nH]1)N1CCN(CC1)[C@H]1CC[C@@H](CC1)c1c[nH]c2ccccc12 |r,wU:19.25,wD:16.18,(-.68,-4.45,;-.69,-6,;.55,-6.91,;.07,-8.37,;.83,-9.72,;.06,-11.05,;-1.49,-11.05,;-2.25,-9.7,;-1.47,-8.37,;-1.94,-6.9,;2.37,-9.72,;3.13,-11.06,;4.67,-11.07,;5.45,-9.74,;4.69,-8.39,;3.15,-8.39,;6.99,-9.75,;7.75,-11.09,;9.28,-11.11,;10.07,-9.78,;9.31,-8.43,;7.76,-8.42,;11.6,-9.79,;12.49,-11.04,;13.95,-10.58,;13.96,-9.05,;15.11,-8.04,;14.81,-6.54,;13.36,-6.05,;12.21,-7.06,;12.52,-8.56,)| Show InChI InChI=1S/C26H31N5/c1-18-28-24-7-4-8-25(26(24)29-18)31-15-13-30(14-16-31)20-11-9-19(10-12-20)22-17-27-23-6-3-2-5-21(22)23/h2-8,17,19-20,27H,9-16H2,1H3,(H,28,29)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252645
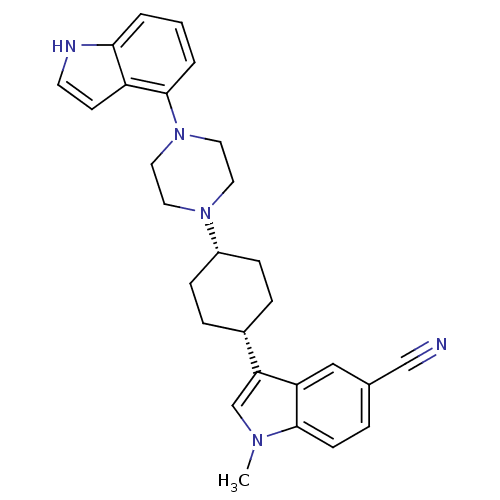
(3-{(1,4-cis)-4-[4-(1H-Indol-4-yl)-piperazin-1-yl]-...)Show SMILES Cn1cc([C@@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(ccc12)C#N |r,wU:7.10,4.3,(-3.26,-11.23,;-4.52,-12.12,;-5.98,-11.63,;-6.89,-12.87,;-8.43,-12.85,;-9.18,-11.51,;-10.73,-11.49,;-11.52,-12.82,;-10.74,-14.16,;-9.21,-14.18,;-13.05,-12.81,;-13.82,-14.14,;-15.37,-14.13,;-16.12,-12.79,;-15.34,-11.46,;-13.81,-11.47,;-17.66,-12.79,;-18.42,-11.45,;-19.95,-11.44,;-20.74,-12.77,;-19.98,-14.12,;-20.45,-15.59,;-19.2,-16.49,;-17.94,-15.58,;-18.43,-14.12,;-6,-14.11,;-6.34,-15.6,;-5.21,-16.64,;-3.74,-16.18,;-3.41,-14.69,;-4.53,-13.66,;-5.55,-18.15,;-5.96,-19.63,)| Show InChI InChI=1S/C28H31N5/c1-31-19-25(24-17-20(18-29)5-10-27(24)31)21-6-8-22(9-7-21)32-13-15-33(16-14-32)28-4-2-3-26-23(28)11-12-30-26/h2-5,10-12,17,19,21-22,30H,6-9,13-16H2,1H3/t21-,22+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252644
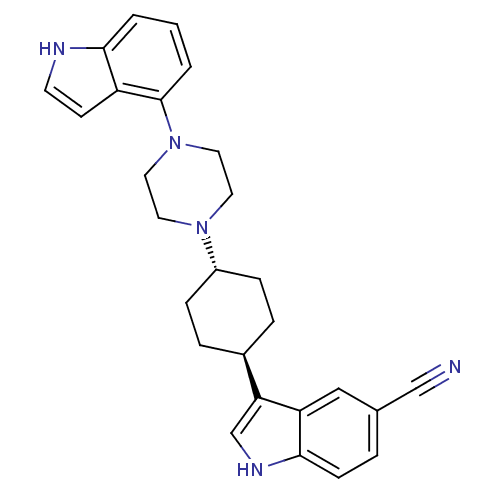
(3-{4-[(1,4-trans)-4-(1H-indol-4-yl)-pipera-zinyl-1...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:9.8,wD:12.15,(31.78,-11.65,;32.19,-10.16,;32.53,-8.66,;33.99,-8.2,;34.33,-6.71,;33.2,-5.67,;33.22,-4.13,;31.76,-3.64,;30.84,-4.88,;29.31,-4.86,;28.52,-6.19,;26.99,-6.18,;26.23,-4.83,;27.01,-3.5,;28.55,-3.52,;24.7,-4.82,;23.92,-6.15,;22.38,-6.14,;21.62,-4.81,;22.4,-3.48,;23.93,-3.48,;20.08,-4.8,;19.32,-3.46,;17.78,-3.45,;17,-4.78,;17.77,-6.13,;17.29,-7.6,;18.54,-8.51,;19.79,-7.6,;19.31,-6.13,;31.74,-6.12,;31.4,-7.62,)| Show InChI InChI=1S/C27H29N5/c28-17-19-4-9-26-23(16-19)24(18-30-26)20-5-7-21(8-6-20)31-12-14-32(15-13-31)27-3-1-2-25-22(27)10-11-29-25/h1-4,9-11,16,18,20-21,29-30H,5-8,12-15H2/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252180
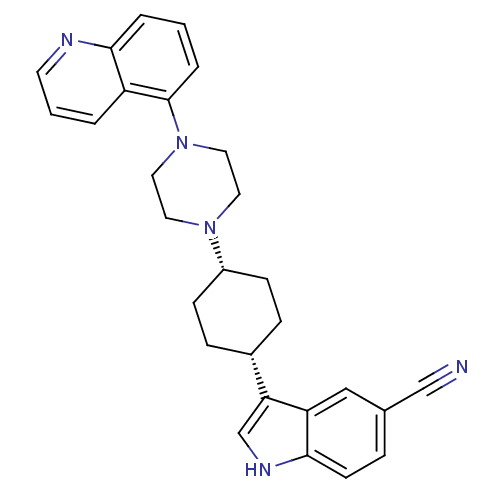
(3-[(1,4-cis)-4-(4-Quinolin-5-yl-piperazin-1-yl)-cy...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:9.8,12.15,(-1.16,-24.06,;-.75,-25.56,;-.44,-27.07,;1.03,-27.57,;1.33,-29.08,;.17,-30.09,;.16,-31.62,;-1.31,-32.08,;-2.2,-30.84,;-3.74,-30.82,;-4.5,-29.48,;-6.05,-29.46,;-6.82,-30.79,;-6.06,-32.13,;-4.53,-32.15,;-8.36,-30.78,;-9.14,-32.11,;-10.68,-32.1,;-11.44,-30.76,;-10.66,-29.43,;-9.12,-29.43,;-12.98,-30.75,;-13.75,-32.09,;-15.3,-32.08,;-16.07,-30.74,;-15.27,-29.41,;-16.04,-28.08,;-15.27,-26.76,;-13.73,-26.77,;-12.98,-28.1,;-13.74,-29.42,;-1.29,-29.59,;-1.59,-28.09,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-27-24(17-20)25(19-31-27)21-7-9-22(10-8-21)32-13-15-33(16-14-32)28-5-1-4-26-23(28)3-2-12-30-26/h1-6,11-12,17,19,21-22,31H,7-10,13-16H2/t21-,22+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252131
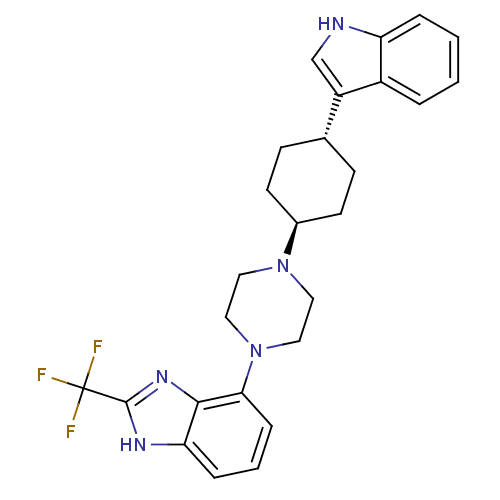
(4-{trans-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CC1)[C@H]1CC[C@@H](CC1)c1c[nH]c2ccccc12 |r,wU:22.28,wD:19.21,(-16.45,-13.54,;-16.45,-15.09,;-14.9,-15.09,;-17.99,-15.1,;-16.45,-16.63,;-15.2,-17.55,;-15.68,-19.01,;-14.93,-20.36,;-15.7,-21.69,;-17.26,-21.69,;-18.02,-20.34,;-17.23,-19,;-17.71,-17.53,;-13.38,-20.36,;-12.63,-21.7,;-11.08,-21.71,;-10.3,-20.38,;-11.07,-19.03,;-12.61,-19.03,;-8.76,-20.39,;-8,-21.73,;-6.47,-21.75,;-5.68,-20.42,;-6.44,-19.07,;-7.99,-19.06,;-4.14,-20.43,;-3.25,-21.68,;-1.79,-21.22,;-1.78,-19.69,;-.63,-18.68,;-.93,-17.18,;-2.39,-16.69,;-3.53,-17.7,;-3.23,-19.19,)| Show InChI InChI=1S/C26H28F3N5/c27-26(28,29)25-31-22-6-3-7-23(24(22)32-25)34-14-12-33(13-15-34)18-10-8-17(9-11-18)20-16-30-21-5-2-1-4-19(20)21/h1-7,16-18,30H,8-15H2,(H,31,32)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252219
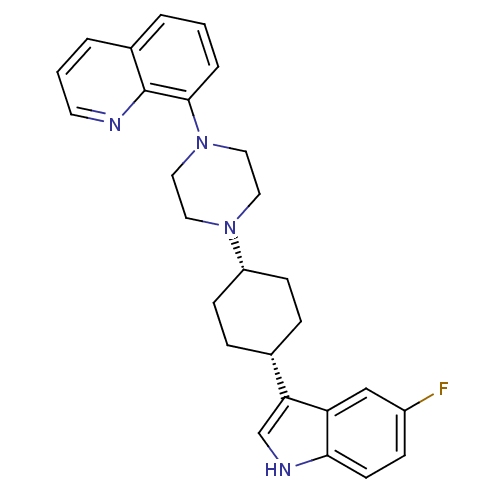
(8-{4-[(1,4-cis)-4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:8.7,11.14,(35.4,-23.67,;35.71,-25.18,;37.17,-25.67,;37.47,-27.18,;36.32,-28.2,;36.3,-29.73,;34.83,-30.19,;33.94,-28.94,;32.4,-28.93,;31.64,-27.59,;30.1,-27.57,;29.33,-28.9,;30.08,-30.24,;31.62,-30.25,;27.79,-28.89,;27.01,-30.22,;25.47,-30.2,;24.71,-28.87,;25.49,-27.54,;27.02,-27.54,;23.17,-28.86,;22.4,-30.19,;20.86,-30.19,;20.09,-28.85,;20.87,-27.52,;20.12,-26.19,;20.88,-24.87,;22.43,-24.87,;23.18,-26.21,;22.41,-27.53,;34.86,-27.7,;34.56,-26.2,)| Show InChI InChI=1S/C27H29FN4/c28-21-8-11-25-23(17-21)24(18-30-25)19-6-9-22(10-7-19)31-13-15-32(16-14-31)26-5-1-3-20-4-2-12-29-27(20)26/h1-5,8,11-12,17-19,22,30H,6-7,9-10,13-16H2/t19-,22+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160613
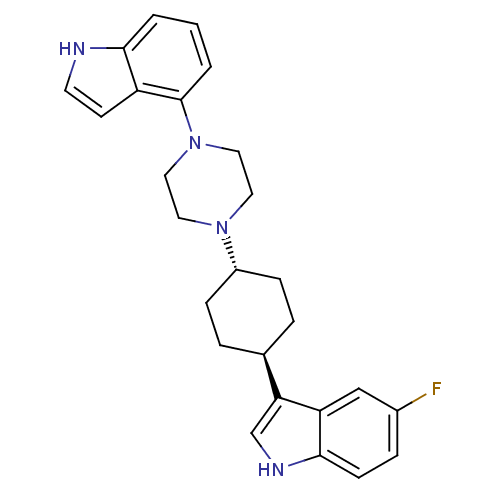
(5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:8.7,wD:11.14,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.69,1.96,;-9.22,1.97,;-9.98,3.31,;-9.21,4.64,;-7.66,4.62,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252181
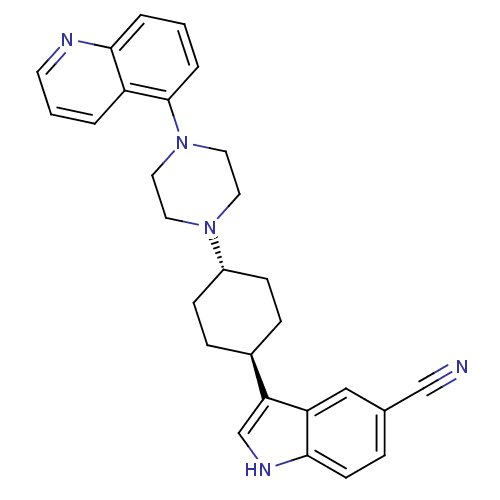
(3-[(1,4-trans)-4-(4-Quinolin-5-yl-piperazin-1-yl)-...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:9.8,wD:12.15,(17.07,-23.33,;17.49,-24.83,;17.79,-26.34,;19.26,-26.83,;19.56,-28.34,;18.4,-29.36,;18.39,-30.89,;16.92,-31.35,;16.03,-30.1,;14.49,-30.09,;13.7,-31.41,;12.17,-31.4,;11.41,-30.06,;12.18,-28.73,;13.73,-28.75,;9.87,-30.05,;9.09,-31.38,;7.55,-31.36,;6.79,-30.03,;7.57,-28.7,;9.11,-28.7,;5.25,-30.02,;4.49,-31.35,;2.95,-31.35,;2.18,-30.01,;2.96,-28.68,;2.2,-27.35,;2.97,-26.03,;4.51,-26.03,;5.27,-27.37,;4.49,-28.69,;16.95,-28.86,;16.64,-27.36,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-27-24(17-20)25(19-31-27)21-7-9-22(10-8-21)32-13-15-33(16-14-32)28-5-1-4-26-23(28)3-2-12-30-26/h1-6,11-12,17,19,21-22,31H,7-10,13-16H2/t21-,22- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252534

(3-[trans-4-[4-(1H-Indol-4-yl)-1-pipera-zinyl]-cycl...)Show SMILES C1C[C@@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]ccc12)c1c[nH]c2ccccc12 |r,wU:2.24,wD:5.6,(8.81,1.57,;10.35,1.56,;11.13,2.89,;10.37,4.23,;8.83,4.24,;8.05,2.92,;6.52,2.93,;5.74,1.6,;4.2,1.61,;3.44,2.94,;4.22,4.27,;5.75,4.27,;1.9,2.95,;1.14,4.29,;-.4,4.3,;-1.18,2.97,;-.41,1.62,;-.89,.15,;.36,-.76,;1.61,.15,;1.13,1.62,;12.66,2.87,;13.58,4.11,;15.04,3.62,;15.03,2.08,;16.15,1.04,;15.82,-.45,;14.35,-.91,;13.22,.13,;13.56,1.62,)| Show InChI InChI=1S/C26H30N4/c1-2-5-24-21(4-1)23(18-28-24)19-8-10-20(11-9-19)29-14-16-30(17-15-29)26-7-3-6-25-22(26)12-13-27-25/h1-7,12-13,18-20,27-28H,8-11,14-17H2/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160603
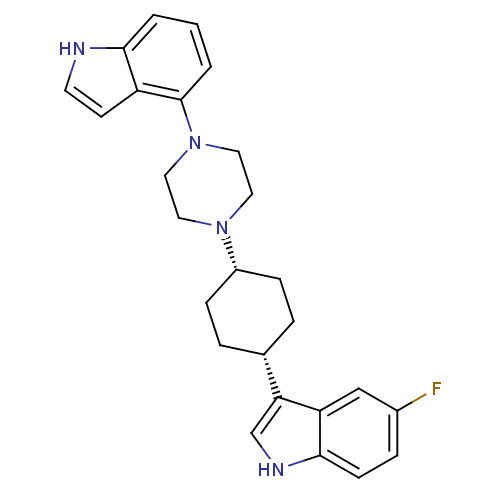
(5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:11.14,8.7,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.66,4.62,;-9.21,4.64,;-9.98,3.31,;-9.22,1.97,;-7.69,1.96,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252588
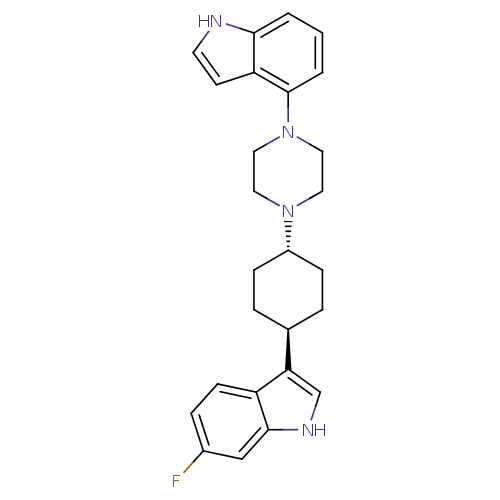
(6-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-pipera-zi...)Show SMILES Fc1ccc2c(c[nH]c2c1)[C@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccc2[nH]ccc12 |r,wU:10.11,wD:13.18,(20.51,-14.6,;19.37,-13.55,;17.91,-14.01,;16.78,-12.97,;17.12,-11.48,;16.22,-10.23,;17.14,-9,;18.6,-9.49,;18.58,-11.03,;19.71,-12.06,;14.69,-10.22,;13.9,-11.54,;12.37,-11.53,;11.61,-10.19,;12.38,-8.86,;13.93,-8.88,;10.07,-10.18,;9.29,-11.51,;7.75,-11.49,;7,-10.16,;7.77,-8.83,;9.31,-8.83,;5.46,-10.16,;4.7,-8.82,;3.16,-8.81,;2.38,-10.14,;3.14,-11.49,;2.67,-12.96,;3.92,-13.86,;5.17,-12.95,;4.69,-11.48,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-21-23(17-29-25(21)16-19)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-22(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252181

(3-[(1,4-trans)-4-(4-Quinolin-5-yl-piperazin-1-yl)-...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:9.8,wD:12.15,(17.07,-23.33,;17.49,-24.83,;17.79,-26.34,;19.26,-26.83,;19.56,-28.34,;18.4,-29.36,;18.39,-30.89,;16.92,-31.35,;16.03,-30.1,;14.49,-30.09,;13.7,-31.41,;12.17,-31.4,;11.41,-30.06,;12.18,-28.73,;13.73,-28.75,;9.87,-30.05,;9.09,-31.38,;7.55,-31.36,;6.79,-30.03,;7.57,-28.7,;9.11,-28.7,;5.25,-30.02,;4.49,-31.35,;2.95,-31.35,;2.18,-30.01,;2.96,-28.68,;2.2,-27.35,;2.97,-26.03,;4.51,-26.03,;5.27,-27.37,;4.49,-28.69,;16.95,-28.86,;16.64,-27.36,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-27-24(17-20)25(19-31-27)21-7-9-22(10-8-21)32-13-15-33(16-14-32)28-5-1-4-26-23(28)3-2-12-30-26/h1-6,11-12,17,19,21-22,31H,7-10,13-16H2/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252220
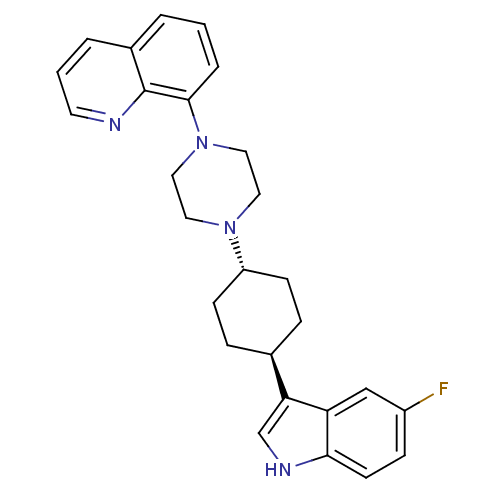
(8-{4-[(1,4-trans)-4-(5-Fluoro-1H-indol-3-yl)-cyclo...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:8.7,wD:11.14,(-6.13,-34.96,;-5.82,-36.47,;-4.36,-36.96,;-4.06,-38.47,;-5.21,-39.48,;-5.23,-41.01,;-6.7,-41.47,;-7.59,-40.23,;-9.13,-40.21,;-9.91,-41.54,;-11.45,-41.52,;-12.21,-40.18,;-11.44,-38.85,;-9.89,-38.87,;-13.75,-40.17,;-14.53,-41.5,;-16.07,-41.48,;-16.82,-40.15,;-16.04,-38.82,;-14.51,-38.82,;-18.36,-40.14,;-19.13,-41.48,;-20.68,-41.47,;-21.45,-40.13,;-20.65,-38.8,;-21.42,-37.47,;-20.65,-36.15,;-19.11,-36.16,;-18.36,-37.49,;-19.12,-38.81,;-6.67,-38.98,;-6.97,-37.48,)| Show InChI InChI=1S/C27H29FN4/c28-21-8-11-25-23(17-21)24(18-30-25)19-6-9-22(10-7-19)31-13-15-32(16-14-31)26-5-1-3-20-4-2-12-29-27(20)26/h1-5,8,11-12,17-19,22,30H,6-7,9-10,13-16H2/t19-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252334
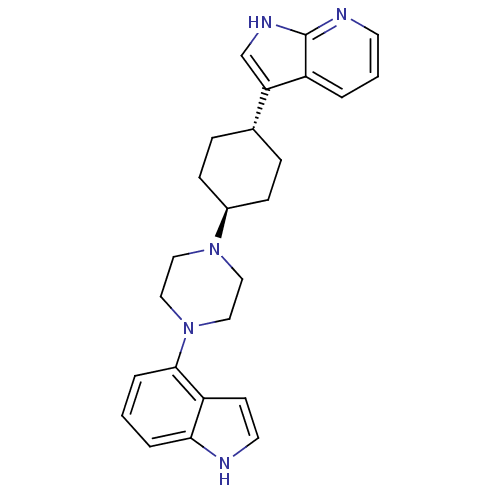
(3-{(1,4-trans)-4-[4-(1H-Indole-4-yl)-piperazin-1-y...)Show SMILES C1C[C@@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]ccc12)c1c[nH]c2ncccc12 |r,wU:2.24,wD:5.6,(30.15,.24,;31.69,.22,;32.48,1.55,;31.72,2.89,;30.17,2.91,;29.4,1.58,;27.85,1.59,;27.08,.26,;25.53,.27,;24.77,1.61,;25.55,2.94,;27.09,2.94,;23.23,1.61,;22.46,.28,;20.92,.28,;20.15,1.63,;20.93,2.96,;20.47,4.43,;21.72,5.32,;22.95,4.41,;22.47,2.95,;34.02,1.53,;34.91,.28,;36.39,.74,;36.4,2.28,;37.56,3.3,;37.26,4.81,;35.79,5.3,;34.64,4.28,;34.94,2.78,)| Show InChI InChI=1S/C25H29N5/c1-4-23-21(10-12-26-23)24(5-1)30-15-13-29(14-16-30)19-8-6-18(7-9-19)22-17-28-25-20(22)3-2-11-27-25/h1-5,10-12,17-19,26H,6-9,13-16H2,(H,27,28)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252083

(4-{cis-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin-1...)Show SMILES C1C[C@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]cnc12)c1c[nH]c2ccccc12 |r,wU:5.6,2.24,(12.86,2.98,;14.41,2.97,;15.17,1.62,;14.38,.29,;12.84,.31,;12.08,1.65,;10.54,1.66,;9.76,.33,;8.22,.34,;7.46,1.68,;8.24,3.01,;9.78,3.01,;5.92,1.68,;5.15,.35,;3.6,.35,;2.84,1.7,;3.62,3.03,;3.15,4.5,;4.4,5.4,;5.65,4.49,;5.16,3.02,;16.7,1.61,;17.59,.36,;19.06,.82,;19.07,2.35,;20.21,3.36,;19.91,4.86,;18.46,5.35,;17.31,4.34,;17.61,2.84,)| Show InChI InChI=1S/C25H29N5/c1-2-5-22-20(4-1)21(16-26-22)18-8-10-19(11-9-18)29-12-14-30(15-13-29)24-7-3-6-23-25(24)28-17-27-23/h1-7,16-19,26H,8-15H2,(H,27,28)/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252179
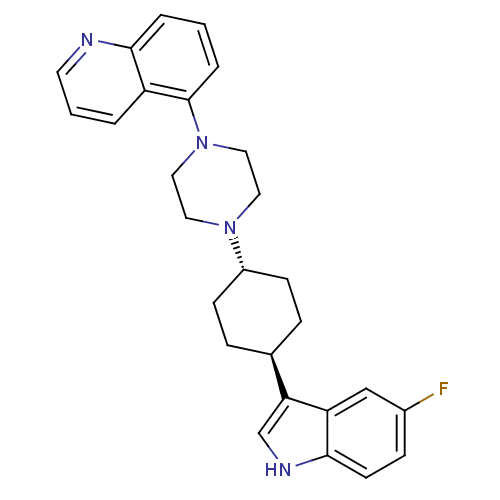
(5-{4-[(1,4-trans)-4-(5-Fluoro-1H-indol-3-yl)-cyclo...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:8.7,wD:11.14,(34.61,-14.19,;34.91,-15.7,;36.38,-16.2,;36.68,-17.71,;35.52,-18.72,;35.51,-20.25,;34.04,-20.71,;33.15,-19.47,;31.61,-19.45,;30.82,-20.78,;29.29,-20.76,;28.53,-19.42,;29.3,-18.09,;30.85,-18.11,;26.99,-19.41,;26.21,-20.74,;24.67,-20.73,;23.91,-19.39,;24.69,-18.06,;26.23,-18.06,;22.37,-19.38,;21.61,-20.72,;20.07,-20.71,;19.3,-19.37,;20.08,-18.04,;19.32,-16.71,;20.09,-15.39,;21.63,-15.4,;22.39,-16.73,;21.61,-18.05,;34.07,-18.22,;33.76,-16.72,)| Show InChI InChI=1S/C27H29FN4/c28-20-8-11-26-23(17-20)24(18-30-26)19-6-9-21(10-7-19)31-13-15-32(16-14-31)27-5-1-4-25-22(27)3-2-12-29-25/h1-5,8,11-12,17-19,21,30H,6-7,9-10,13-16H2/t19-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252644

(3-{4-[(1,4-trans)-4-(1H-indol-4-yl)-pipera-zinyl-1...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:9.8,wD:12.15,(31.78,-11.65,;32.19,-10.16,;32.53,-8.66,;33.99,-8.2,;34.33,-6.71,;33.2,-5.67,;33.22,-4.13,;31.76,-3.64,;30.84,-4.88,;29.31,-4.86,;28.52,-6.19,;26.99,-6.18,;26.23,-4.83,;27.01,-3.5,;28.55,-3.52,;24.7,-4.82,;23.92,-6.15,;22.38,-6.14,;21.62,-4.81,;22.4,-3.48,;23.93,-3.48,;20.08,-4.8,;19.32,-3.46,;17.78,-3.45,;17,-4.78,;17.77,-6.13,;17.29,-7.6,;18.54,-8.51,;19.79,-7.6,;19.31,-6.13,;31.74,-6.12,;31.4,-7.62,)| Show InChI InChI=1S/C27H29N5/c28-17-19-4-9-26-23(16-19)24(18-30-26)20-5-7-21(8-6-20)31-12-14-32(15-13-31)27-3-1-2-25-22(27)10-11-29-25/h1-4,9-11,16,18,20-21,29-30H,5-8,12-15H2/t20-,21- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252279
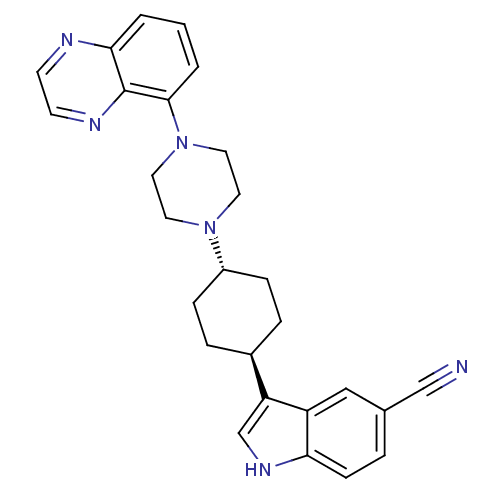
(3-[(1,4-trans)-4-(4-Quinoxalin-5-yl-piperazin-1-yl...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4nccnc34)c2c1 |r,wU:9.8,wD:12.15,(12.55,-43.28,;12.96,-44.78,;13.27,-46.29,;14.73,-46.78,;15.03,-48.29,;13.88,-49.31,;13.86,-50.84,;12.39,-51.3,;11.5,-50.05,;9.96,-50.04,;9.18,-51.36,;7.64,-51.35,;6.89,-50,;7.66,-48.68,;9.2,-48.69,;5.35,-49.99,;4.57,-51.32,;3.03,-51.31,;2.27,-49.98,;3.05,-48.65,;4.59,-48.65,;.73,-49.97,;-.04,-51.3,;-1.57,-51.3,;-2.34,-49.96,;-1.56,-48.63,;-2.32,-47.3,;-1.55,-45.98,;-.01,-45.98,;.75,-47.32,;-.03,-48.64,;12.42,-48.81,;12.12,-47.31,)| Show InChI InChI=1S/C27H28N6/c28-17-19-4-9-24-22(16-19)23(18-31-24)20-5-7-21(8-6-20)32-12-14-33(15-13-32)26-3-1-2-25-27(26)30-11-10-29-25/h1-4,9-11,16,18,20-21,31H,5-8,12-15H2/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252128
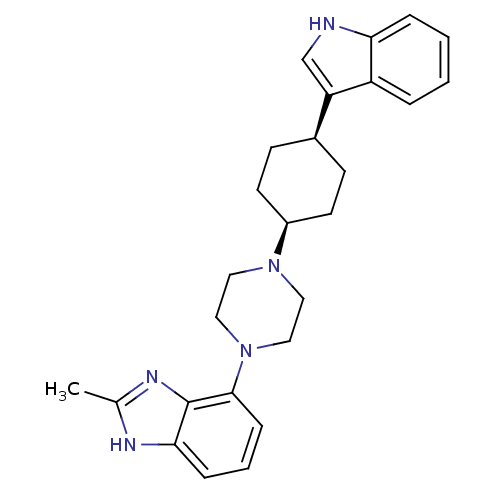
(4-{cis-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin-1...)Show SMILES Cc1nc2c(cccc2[nH]1)N1CCN(CC1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccccc12 |r,wU:16.18,19.25,(-18.8,-3.58,;-18.81,-5.12,;-17.56,-6.03,;-18.04,-7.5,;-17.29,-8.84,;-18.06,-10.17,;-19.61,-10.17,;-20.38,-8.82,;-19.58,-7.49,;-20.06,-6.02,;-15.74,-8.85,;-14.99,-10.18,;-13.44,-10.19,;-12.66,-8.86,;-13.43,-7.52,;-14.96,-7.51,;-11.12,-8.87,;-10.34,-7.54,;-8.79,-7.56,;-8.03,-8.9,;-8.82,-10.23,;-10.36,-10.22,;-6.5,-8.92,;-5.61,-10.16,;-4.14,-9.7,;-4.13,-8.17,;-2.99,-7.17,;-3.29,-5.66,;-4.74,-5.18,;-5.89,-6.19,;-5.59,-7.68,)| Show InChI InChI=1S/C26H31N5/c1-18-28-24-7-4-8-25(26(24)29-18)31-15-13-30(14-16-31)20-11-9-19(10-12-20)22-17-27-23-6-3-2-5-21(22)23/h2-8,17,19-20,27H,9-16H2,1H3,(H,28,29)/t19-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252535

(5-Fluoro-3-{cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Cn1cc([C@@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(F)ccc12 |r,wU:7.10,4.3,(34.32,5.15,;33.06,4.26,;31.6,4.75,;30.68,3.52,;29.15,3.53,;28.39,4.87,;26.85,4.89,;26.07,3.56,;26.83,2.22,;28.36,2.2,;24.53,3.57,;23.76,2.24,;22.22,2.26,;21.46,3.59,;22.24,4.92,;23.77,4.92,;19.92,3.59,;19.16,4.93,;17.62,4.94,;16.84,3.61,;17.61,2.26,;17.13,.79,;18.38,-.11,;19.63,.8,;19.15,2.26,;31.58,2.27,;31.24,.78,;32.36,-.26,;32.03,-1.77,;33.83,.2,;34.17,1.69,;33.04,2.72,)| Show InChI InChI=1S/C27H31FN4/c1-30-18-24(23-17-20(28)7-10-26(23)30)19-5-8-21(9-6-19)31-13-15-32(16-14-31)27-4-2-3-25-22(27)11-12-29-25/h2-4,7,10-12,17-19,21,29H,5-6,8-9,13-16H2,1H3/t19-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252537
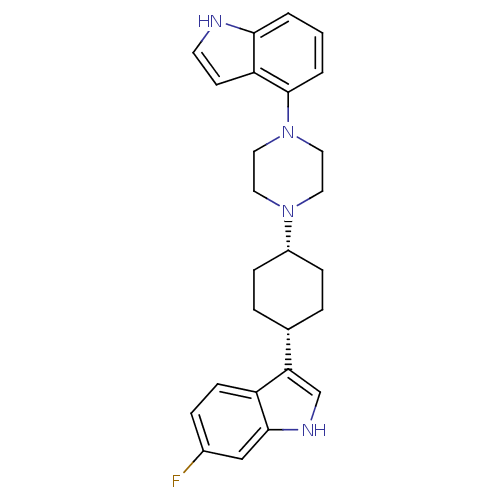
(6-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2c(c[nH]c2c1)[C@@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccc2[nH]ccc12 |r,wU:13.18,10.11,(20.51,-14.6,;19.37,-13.55,;17.91,-14.01,;16.78,-12.97,;17.12,-11.48,;16.22,-10.23,;17.14,-9,;18.6,-9.49,;18.58,-11.03,;19.71,-12.06,;14.69,-10.22,;13.93,-8.88,;12.38,-8.86,;11.61,-10.19,;12.37,-11.53,;13.9,-11.54,;10.07,-10.18,;9.29,-11.51,;7.75,-11.49,;7,-10.16,;7.77,-8.83,;9.31,-8.83,;5.46,-10.16,;4.7,-8.82,;3.16,-8.81,;2.38,-10.14,;3.14,-11.49,;2.67,-12.96,;3.92,-13.86,;5.17,-12.95,;4.69,-11.48,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-21-23(17-29-25(21)16-19)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-22(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252083

(4-{cis-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin-1...)Show SMILES C1C[C@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]cnc12)c1c[nH]c2ccccc12 |r,wU:5.6,2.24,(12.86,2.98,;14.41,2.97,;15.17,1.62,;14.38,.29,;12.84,.31,;12.08,1.65,;10.54,1.66,;9.76,.33,;8.22,.34,;7.46,1.68,;8.24,3.01,;9.78,3.01,;5.92,1.68,;5.15,.35,;3.6,.35,;2.84,1.7,;3.62,3.03,;3.15,4.5,;4.4,5.4,;5.65,4.49,;5.16,3.02,;16.7,1.61,;17.59,.36,;19.06,.82,;19.07,2.35,;20.21,3.36,;19.91,4.86,;18.46,5.35,;17.31,4.34,;17.61,2.84,)| Show InChI InChI=1S/C25H29N5/c1-2-5-22-20(4-1)21(16-26-22)18-8-10-19(11-9-18)29-12-14-30(15-13-29)24-7-3-6-23-25(24)28-17-27-23/h1-7,16-19,26H,8-15H2,(H,27,28)/t18-,19+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252082
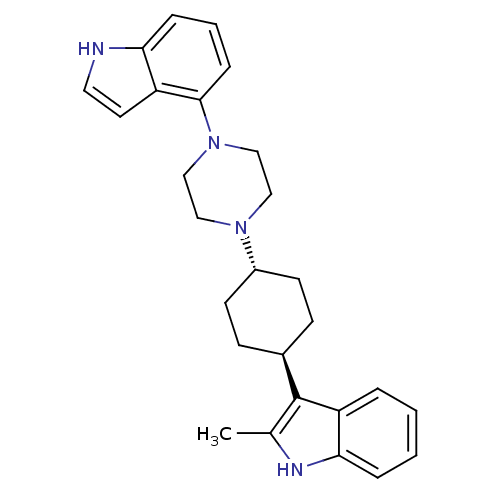
(3-[trans-4-[4-(1H-Indol-4-yl)-1-pipera-zinyl]-cycl...)Show SMILES Cc1[nH]c2ccccc2c1[C@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccc2[nH]ccc12 |r,wU:10.11,wD:13.18,(-8.67,-34.74,;-8.21,-36.21,;-6.75,-36.7,;-6.76,-38.24,;-5.64,-39.27,;-5.98,-40.76,;-7.44,-41.22,;-8.57,-40.18,;-8.23,-38.69,;-9.13,-37.44,;-10.66,-37.43,;-11.45,-38.75,;-12.99,-38.74,;-13.75,-37.4,;-12.97,-36.07,;-11.42,-36.09,;-15.28,-37.39,;-16.06,-38.72,;-17.6,-38.7,;-18.35,-37.37,;-17.57,-36.04,;-16.04,-36.04,;-19.89,-37.37,;-20.66,-36.03,;-22.18,-36.02,;-22.97,-37.35,;-22.21,-38.7,;-22.69,-40.17,;-21.43,-41.07,;-20.18,-40.16,;-20.66,-38.69,)| Show InChI InChI=1S/C27H32N4/c1-19-27(23-5-2-3-6-25(23)29-19)20-9-11-21(12-10-20)30-15-17-31(18-16-30)26-8-4-7-24-22(26)13-14-28-24/h2-8,13-14,20-21,28-29H,9-12,15-18H2,1H3/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252647

(1-Ethyl-3-{(1,4-cis)-4-[4-(1H-indol-4-yl)-piperazi...)Show SMILES CCn1cc([C@@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(ccc12)C#N |r,wU:8.11,5.4,(16.98,-13.21,;15.58,-12.57,;14.32,-13.46,;12.86,-12.97,;11.95,-14.21,;10.41,-14.19,;9.66,-12.85,;8.11,-12.83,;7.34,-14.16,;8.09,-15.5,;9.63,-15.52,;5.8,-14.15,;5.02,-15.48,;3.48,-15.47,;2.72,-14.13,;3.5,-12.8,;5.04,-12.81,;1.19,-14.13,;.43,-12.79,;-1.11,-12.78,;-1.89,-14.11,;-1.13,-15.46,;-1.6,-16.93,;-.35,-17.84,;.9,-16.93,;.42,-15.46,;12.84,-15.45,;12.5,-16.94,;13.63,-17.99,;15.1,-17.52,;15.43,-16.03,;14.31,-15,;13.29,-19.49,;12.88,-20.97,)| Show InChI InChI=1S/C29H33N5/c1-2-32-20-26(25-18-21(19-30)6-11-29(25)32)22-7-9-23(10-8-22)33-14-16-34(17-15-33)28-5-3-4-27-24(28)12-13-31-27/h3-6,11-13,18,20,22-23,31H,2,7-10,14-17H2,1H3/t22-,23+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252279

(3-[(1,4-trans)-4-(4-Quinoxalin-5-yl-piperazin-1-yl...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4nccnc34)c2c1 |r,wU:9.8,wD:12.15,(12.55,-43.28,;12.96,-44.78,;13.27,-46.29,;14.73,-46.78,;15.03,-48.29,;13.88,-49.31,;13.86,-50.84,;12.39,-51.3,;11.5,-50.05,;9.96,-50.04,;9.18,-51.36,;7.64,-51.35,;6.89,-50,;7.66,-48.68,;9.2,-48.69,;5.35,-49.99,;4.57,-51.32,;3.03,-51.31,;2.27,-49.98,;3.05,-48.65,;4.59,-48.65,;.73,-49.97,;-.04,-51.3,;-1.57,-51.3,;-2.34,-49.96,;-1.56,-48.63,;-2.32,-47.3,;-1.55,-45.98,;-.01,-45.98,;.75,-47.32,;-.03,-48.64,;12.42,-48.81,;12.12,-47.31,)| Show InChI InChI=1S/C27H28N6/c28-17-19-4-9-24-22(16-19)23(18-31-24)20-5-7-21(8-6-20)32-12-14-33(15-13-32)26-3-1-2-25-27(26)30-11-10-29-25/h1-4,9-11,16,18,20-21,31H,5-8,12-15H2/t20-,21- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252178

(5-{4-[(1,4-cis)-4-(5-Fluoro-1H-indol-3-yl)-cyclohe...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:8.7,11.14,(15.69,-14.56,;15.99,-16.07,;17.46,-16.56,;17.76,-18.07,;16.6,-19.08,;16.59,-20.62,;15.12,-21.08,;14.23,-19.83,;12.69,-19.81,;11.93,-18.47,;10.38,-18.45,;9.61,-19.78,;10.37,-21.12,;11.9,-21.14,;8.07,-19.77,;7.29,-21.1,;5.75,-21.09,;5,-19.76,;5.77,-18.43,;7.31,-18.43,;3.45,-19.75,;2.69,-21.08,;1.15,-21.08,;.38,-19.73,;1.16,-18.4,;.4,-17.08,;1.17,-15.75,;2.71,-15.76,;3.47,-17.09,;2.69,-18.41,;15.15,-18.59,;14.84,-17.08,)| Show InChI InChI=1S/C27H29FN4/c28-20-8-11-26-23(17-20)24(18-30-26)19-6-9-21(10-7-19)31-13-15-32(16-14-31)27-5-1-4-25-22(27)3-2-12-29-25/h1-5,8,11-12,17-19,21,30H,6-7,9-10,13-16H2/t19-,21+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252333
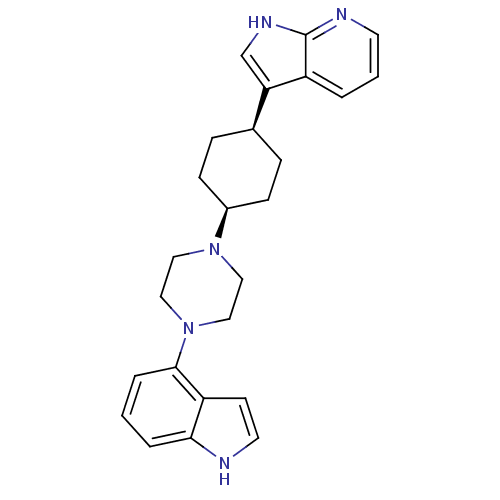
(3-{(1,4-cis)-4-[(1H-Indole-4-yl)-piperazin-1-yl]-c...)Show SMILES C1C[C@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]ccc12)c1c[nH]c2ncccc12 |r,wU:5.6,2.24,(11.14,2.18,;12.69,2.16,;13.45,.82,;12.67,-.51,;11.13,-.5,;10.37,.85,;8.83,.86,;8.05,-.47,;6.51,-.46,;5.75,.87,;6.52,2.21,;8.06,2.2,;4.2,.88,;3.43,-.45,;1.89,-.45,;1.12,.9,;1.9,2.23,;1.44,3.7,;2.69,4.59,;3.93,3.68,;3.44,2.22,;15,.8,;15.89,-.45,;17.36,.01,;17.38,1.55,;18.53,2.56,;18.23,4.08,;16.76,4.57,;15.61,3.55,;15.92,2.05,)| Show InChI InChI=1S/C25H29N5/c1-4-23-21(10-12-26-23)24(5-1)30-15-13-29(14-16-30)19-8-6-18(7-9-19)22-17-28-25-20(22)3-2-11-27-25/h1-5,10-12,17-19,26H,6-9,13-16H2,(H,27,28)/t18-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252130
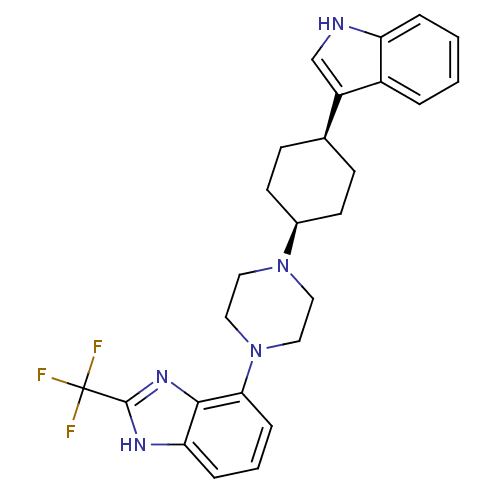
(4-{cis-4-[4-(1H-Indol-3-yl)cyclohexyl]-piperazin-1...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CC1)[C@H]1CC[C@H](CC1)c1c[nH]c2ccccc12 |r,wU:19.21,22.28,(18.54,-3.2,;18.56,-4.75,;20.1,-4.75,;17.01,-4.76,;18.55,-6.29,;19.79,-7.21,;19.3,-8.67,;20.07,-10.01,;19.29,-11.34,;17.75,-11.34,;16.98,-9.99,;17.76,-8.66,;17.29,-7.19,;21.6,-10.02,;22.36,-11.35,;23.91,-11.36,;24.68,-10.03,;23.92,-8.69,;22.38,-8.69,;26.22,-10.04,;27,-8.71,;28.55,-8.73,;29.31,-10.07,;28.52,-11.4,;26.98,-11.39,;30.84,-10.09,;31.73,-11.33,;33.19,-10.87,;33.2,-9.35,;34.35,-8.34,;34.05,-6.84,;32.59,-6.35,;31.45,-7.36,;31.75,-8.85,)| Show InChI InChI=1S/C26H28F3N5/c27-26(28,29)25-31-22-6-3-7-23(24(22)32-25)34-14-12-33(13-15-34)18-10-8-17(9-11-18)20-16-30-21-5-2-1-4-19(20)21/h1-7,16-18,30H,8-15H2,(H,31,32)/t17-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252222

(3-[(1,4-trans)-4-(4-Quinolin-8-yl-piperazin-1-yl)-...)Show SMILES N#Cc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:12.15,wD:9.8,(34.65,-31.84,;35.06,-33.34,;35.37,-34.85,;36.83,-35.34,;37.13,-36.85,;35.98,-37.86,;35.96,-39.4,;34.49,-39.86,;33.6,-38.61,;32.06,-38.59,;31.3,-37.25,;29.76,-37.23,;28.98,-38.56,;29.74,-39.91,;31.27,-39.92,;27.44,-38.55,;26.67,-39.88,;25.13,-39.87,;24.37,-38.54,;25.14,-37.21,;26.68,-37.21,;22.83,-38.53,;22.06,-39.86,;20.52,-39.86,;19.75,-38.51,;20.53,-37.18,;19.77,-35.86,;20.54,-34.53,;22.08,-34.54,;22.84,-35.87,;22.06,-37.2,;34.52,-37.37,;34.22,-35.86,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-26-24(17-20)25(19-31-26)21-7-9-23(10-8-21)32-13-15-33(16-14-32)27-5-1-3-22-4-2-12-30-28(22)27/h1-6,11-12,17,19,21,23,31H,7-10,13-16H2/t21-,23- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252590

(4-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...)Show SMILES Fc1cccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c12 |r,wU:12.15,wD:9.8,(10.58,-8.48,;12.05,-8.02,;13.17,-9.06,;14.64,-8.6,;14.97,-7.11,;13.85,-6.08,;13.87,-4.54,;12.41,-4.05,;11.49,-5.28,;9.96,-5.27,;9.2,-3.93,;7.65,-3.91,;6.88,-5.24,;7.64,-6.58,;9.17,-6.59,;5.34,-5.23,;4.56,-6.56,;3.02,-6.54,;2.26,-5.21,;3.04,-3.88,;4.58,-3.88,;.73,-5.21,;-.03,-3.87,;-1.57,-3.86,;-2.35,-5.19,;-1.59,-6.54,;-2.06,-8.01,;-.81,-8.91,;.44,-8,;-.04,-6.53,;12.39,-6.53,)| Show InChI InChI=1S/C26H29FN4/c27-22-3-1-5-24-26(22)21(17-29-24)18-7-9-19(10-8-18)30-13-15-31(16-14-30)25-6-2-4-23-20(25)11-12-28-23/h1-6,11-12,17-19,28-29H,7-10,13-16H2/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50251879

(3-{(1,4-cis)-4-[4-(1H-Indol-4-yl)-piperazin-1-yl]-...)Show SMILES CCCn1cc([C@@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(ccc12)C#N |r,wU:9.12,6.5,(39.98,-12.38,;38.72,-13.27,;37.32,-12.63,;36.06,-13.52,;34.6,-13.03,;33.69,-14.27,;32.15,-14.25,;31.4,-12.91,;29.85,-12.89,;29.08,-14.22,;29.84,-15.56,;31.37,-15.58,;27.54,-14.21,;26.76,-15.54,;25.22,-15.53,;24.46,-14.2,;25.24,-12.87,;26.78,-12.87,;22.93,-14.19,;22.17,-12.85,;20.63,-12.84,;19.85,-14.17,;20.61,-15.52,;20.14,-16.99,;21.39,-17.9,;22.64,-16.99,;22.16,-15.52,;34.58,-15.51,;34.24,-17,;35.37,-18.05,;36.84,-17.59,;37.17,-16.09,;36.05,-15.06,;35.03,-19.55,;34.62,-21.03,)| Show InChI InChI=1S/C30H35N5/c1-2-14-35-21-27(26-19-22(20-31)6-11-30(26)35)23-7-9-24(10-8-23)33-15-17-34(18-16-33)29-5-3-4-28-25(29)12-13-32-28/h3-6,11-13,19,21,23-24,32H,2,7-10,14-18H2,1H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252491

(3-[cis-4-[4-(1H-Indol-4-yl)-1-piperazinyl]-cyclo-h...)Show SMILES C1C[C@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]ccc12)c1c[nH]c2ccccc12 |r,wU:5.6,2.24,(8.83,4.24,;10.37,4.23,;11.13,2.89,;10.35,1.56,;8.81,1.57,;8.05,2.92,;6.52,2.93,;5.74,1.6,;4.2,1.61,;3.44,2.94,;4.22,4.27,;5.75,4.27,;1.9,2.95,;1.14,4.29,;-.4,4.3,;-1.18,2.97,;-.41,1.62,;-.89,.15,;.36,-.76,;1.61,.15,;1.13,1.62,;12.66,2.87,;13.58,4.11,;15.04,3.62,;15.03,2.08,;16.15,1.04,;15.82,-.45,;14.35,-.91,;13.22,.13,;13.56,1.62,)| Show InChI InChI=1S/C26H30N4/c1-2-5-24-21(4-1)23(18-28-24)19-8-10-20(11-9-19)29-14-16-30(17-15-29)26-7-3-6-25-22(26)12-13-27-25/h1-7,12-13,18-20,27-28H,8-11,14-17H2/t19-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252537

(6-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2c(c[nH]c2c1)[C@@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccc2[nH]ccc12 |r,wU:13.18,10.11,(20.51,-14.6,;19.37,-13.55,;17.91,-14.01,;16.78,-12.97,;17.12,-11.48,;16.22,-10.23,;17.14,-9,;18.6,-9.49,;18.58,-11.03,;19.71,-12.06,;14.69,-10.22,;13.93,-8.88,;12.38,-8.86,;11.61,-10.19,;12.37,-11.53,;13.9,-11.54,;10.07,-10.18,;9.29,-11.51,;7.75,-11.49,;7,-10.16,;7.77,-8.83,;9.31,-8.83,;5.46,-10.16,;4.7,-8.82,;3.16,-8.81,;2.38,-10.14,;3.14,-11.49,;2.67,-12.96,;3.92,-13.86,;5.17,-12.95,;4.69,-11.48,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-21-23(17-29-25(21)16-19)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-22(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50160603

(5-Fluoro-3-[cis-4-[4-(1H-indol-4-yl)-1-piperazinyl...)Show SMILES Fc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:11.14,8.7,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.66,4.62,;-9.21,4.64,;-9.98,3.31,;-9.22,1.97,;-7.69,1.96,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252491

(3-[cis-4-[4-(1H-Indol-4-yl)-1-piperazinyl]-cyclo-h...)Show SMILES C1C[C@H](CC[C@H]1N1CCN(CC1)c1cccc2[nH]ccc12)c1c[nH]c2ccccc12 |r,wU:5.6,2.24,(8.83,4.24,;10.37,4.23,;11.13,2.89,;10.35,1.56,;8.81,1.57,;8.05,2.92,;6.52,2.93,;5.74,1.6,;4.2,1.61,;3.44,2.94,;4.22,4.27,;5.75,4.27,;1.9,2.95,;1.14,4.29,;-.4,4.3,;-1.18,2.97,;-.41,1.62,;-.89,.15,;.36,-.76,;1.61,.15,;1.13,1.62,;12.66,2.87,;13.58,4.11,;15.04,3.62,;15.03,2.08,;16.15,1.04,;15.82,-.45,;14.35,-.91,;13.22,.13,;13.56,1.62,)| Show InChI InChI=1S/C26H30N4/c1-2-5-24-21(4-1)23(18-28-24)19-8-10-20(11-9-19)29-14-16-30(17-15-29)26-7-3-6-25-22(26)12-13-27-25/h1-7,12-13,18-20,27-28H,8-11,14-17H2/t19-,20+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50251881

(3-{(1,4-cis)-4-[4-(1H-Indol-4-yl)-piperazin-1-yl]-...)Show SMILES CC(C)n1cc([C@@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(ccc12)C#N |r,wU:9.12,6.5,(-3.33,-24.68,;-4.73,-24.04,;-4.88,-22.51,;-5.99,-24.94,;-7.45,-24.44,;-8.36,-25.68,;-9.89,-25.67,;-10.65,-24.32,;-12.21,-24.31,;-12.99,-25.64,;-12.22,-26.98,;-10.68,-26.99,;-14.52,-25.62,;-15.29,-26.95,;-16.83,-26.94,;-17.58,-25.61,;-16.81,-24.28,;-15.28,-24.28,;-19.13,-25.61,;-19.89,-24.27,;-21.42,-24.26,;-22.21,-25.59,;-21.45,-26.93,;-21.92,-28.4,;-20.67,-29.31,;-19.41,-28.4,;-19.9,-26.93,;-7.47,-26.93,;-7.81,-28.42,;-6.68,-29.46,;-5.21,-29,;-4.88,-27.51,;-6,-26.48,;-7.02,-30.96,;-7.43,-32.45,)| Show InChI InChI=1S/C30H35N5/c1-21(2)35-20-27(26-18-22(19-31)6-11-30(26)35)23-7-9-24(10-8-23)33-14-16-34(17-15-33)29-5-3-4-28-25(29)12-13-32-28/h3-6,11-13,18,20-21,23-24,32H,7-10,14-17H2,1-2H3/t23-,24+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50252220

(8-{4-[(1,4-trans)-4-(5-Fluoro-1H-indol-3-yl)-cyclo...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:8.7,wD:11.14,(-6.13,-34.96,;-5.82,-36.47,;-4.36,-36.96,;-4.06,-38.47,;-5.21,-39.48,;-5.23,-41.01,;-6.7,-41.47,;-7.59,-40.23,;-9.13,-40.21,;-9.91,-41.54,;-11.45,-41.52,;-12.21,-40.18,;-11.44,-38.85,;-9.89,-38.87,;-13.75,-40.17,;-14.53,-41.5,;-16.07,-41.48,;-16.82,-40.15,;-16.04,-38.82,;-14.51,-38.82,;-18.36,-40.14,;-19.13,-41.48,;-20.68,-41.47,;-21.45,-40.13,;-20.65,-38.8,;-21.42,-37.47,;-20.65,-36.15,;-19.11,-36.16,;-18.36,-37.49,;-19.12,-38.81,;-6.67,-38.98,;-6.97,-37.48,)| Show InChI InChI=1S/C27H29FN4/c28-21-8-11-25-23(17-21)24(18-30-25)19-6-9-22(10-7-19)31-13-15-32(16-14-31)26-5-1-3-20-4-2-12-29-27(20)26/h1-5,8,11-12,17-19,22,30H,6-7,9-10,13-16H2/t19-,22- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252281
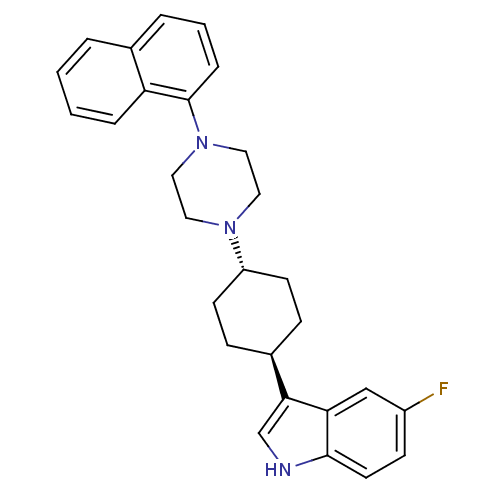
(5-Fluoro-3-[(1,4-trans)-4-(4-naphthalen-1-yl-piper...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ccccc34)c2c1 |r,wU:8.7,wD:11.14,(-1.55,5.95,;-1.24,4.44,;.22,3.95,;.52,2.44,;-.63,1.43,;-.64,-.11,;-2.12,-.57,;-3.01,.68,;-4.55,.7,;-5.33,-.63,;-6.87,-.61,;-7.62,.73,;-6.85,2.06,;-5.31,2.04,;-9.16,.74,;-9.94,-.59,;-11.49,-.58,;-12.24,.75,;-11.46,2.09,;-9.93,2.08,;-13.78,.76,;-14.56,-.57,;-16.1,-.56,;-16.87,.78,;-16.08,2.11,;-16.85,3.44,;-16.07,4.76,;-14.53,4.75,;-13.78,3.42,;-14.55,2.1,;-2.09,1.93,;-2.39,3.43,)| Show InChI InChI=1S/C28H30FN3/c29-22-10-13-27-25(18-22)26(19-30-27)21-8-11-23(12-9-21)31-14-16-32(17-15-31)28-7-3-5-20-4-1-2-6-24(20)28/h1-7,10,13,18-19,21,23,30H,8-9,11-12,14-17H2/t21-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252221

(3-[(1,4-cis)-4-(4-Quinolin-8-yl-piperazin-1-yl)-cy...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4cccnc34)c2c1 |r,wU:9.8,12.15,(14.01,-32.59,;14.42,-34.09,;14.73,-35.6,;16.19,-36.09,;16.49,-37.6,;15.34,-38.61,;15.33,-40.15,;13.85,-40.61,;12.96,-39.36,;11.42,-39.34,;10.66,-38,;9.12,-37.98,;8.35,-39.31,;9.1,-40.66,;10.64,-40.67,;6.81,-39.3,;6.03,-40.63,;4.49,-40.62,;3.73,-39.29,;4.51,-37.96,;6.04,-37.96,;2.19,-39.28,;1.42,-40.61,;-.12,-40.61,;-.89,-39.26,;-.11,-37.93,;-.87,-36.61,;-.1,-35.28,;1.44,-35.29,;2.2,-36.62,;1.43,-37.95,;13.88,-38.12,;13.58,-36.61,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-26-24(17-20)25(19-31-26)21-7-9-23(10-8-21)32-13-15-33(16-14-32)27-5-1-3-22-4-2-12-30-28(22)27/h1-6,11-12,17,19,21,23,31H,7-10,13-16H2/t21-,23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160613

(5-Fluoro-3-[trans-4-[4-(1H-indol-4-yl)-1-piperazin...)Show SMILES Fc1ccc2[nH]cc([C@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4[nH]ccc34)c2c1 |r,wU:8.7,wD:11.14,(-4.03,-2.02,;-3.69,-.51,;-2.22,-.05,;-1.89,1.44,;-3.01,2.47,;-2.99,4.01,;-4.45,4.5,;-5.37,3.27,;-6.9,3.28,;-7.69,1.96,;-9.22,1.97,;-9.98,3.31,;-9.21,4.64,;-7.66,4.62,;-11.52,3.32,;-12.3,1.99,;-13.84,2.01,;-14.59,3.34,;-13.81,4.67,;-12.29,4.67,;-16.14,3.34,;-16.9,4.68,;-18.43,4.69,;-19.22,3.36,;-18.46,2.01,;-18.93,.54,;-17.68,-.36,;-16.42,.55,;-16.91,2.02,;-4.47,2.02,;-4.81,.53,)| Show InChI InChI=1S/C26H29FN4/c27-19-6-9-25-22(16-19)23(17-29-25)18-4-7-20(8-5-18)30-12-14-31(15-13-30)26-3-1-2-24-21(26)10-11-28-24/h1-3,6,9-11,16-18,20,28-29H,4-5,7-8,12-15H2/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from rat cortical 5HTT reuptake site |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252646
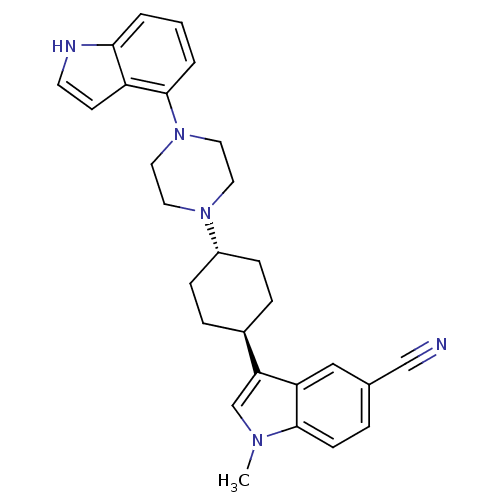
(3-{(1,4-trans)-4-[4-(1H-Indol-4-yl)-piperazin-1-yl...)Show SMILES Cn1cc([C@H]2CC[C@@H](CC2)N2CCN(CC2)c2cccc3[nH]ccc23)c2cc(ccc12)C#N |r,wU:4.3,wD:7.10,(-3.26,-11.23,;-4.52,-12.12,;-5.98,-11.63,;-6.89,-12.87,;-8.43,-12.85,;-9.21,-14.18,;-10.74,-14.16,;-11.52,-12.82,;-10.73,-11.49,;-9.18,-11.51,;-13.05,-12.81,;-13.82,-14.14,;-15.37,-14.13,;-16.12,-12.79,;-15.34,-11.46,;-13.81,-11.47,;-17.66,-12.79,;-18.42,-11.45,;-19.95,-11.44,;-20.74,-12.77,;-19.98,-14.12,;-20.45,-15.59,;-19.2,-16.49,;-17.94,-15.58,;-18.43,-14.12,;-6,-14.11,;-6.34,-15.6,;-5.21,-16.64,;-3.74,-16.18,;-3.41,-14.69,;-4.53,-13.66,;-5.55,-18.15,;-5.96,-19.63,)| Show InChI InChI=1S/C28H31N5/c1-31-19-25(24-17-20(18-29)5-10-27(24)31)21-6-8-22(9-7-21)32-13-15-33(16-14-32)28-4-2-3-26-23(28)11-12-30-26/h2-5,10-12,17,19,21-22,30H,6-9,13-16H2,1H3/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252180

(3-[(1,4-cis)-4-(4-Quinolin-5-yl-piperazin-1-yl)-cy...)Show SMILES N#Cc1ccc2[nH]cc([C@@H]3CC[C@@H](CC3)N3CCN(CC3)c3cccc4ncccc34)c2c1 |r,wU:9.8,12.15,(-1.16,-24.06,;-.75,-25.56,;-.44,-27.07,;1.03,-27.57,;1.33,-29.08,;.17,-30.09,;.16,-31.62,;-1.31,-32.08,;-2.2,-30.84,;-3.74,-30.82,;-4.5,-29.48,;-6.05,-29.46,;-6.82,-30.79,;-6.06,-32.13,;-4.53,-32.15,;-8.36,-30.78,;-9.14,-32.11,;-10.68,-32.1,;-11.44,-30.76,;-10.66,-29.43,;-9.12,-29.43,;-12.98,-30.75,;-13.75,-32.09,;-15.3,-32.08,;-16.07,-30.74,;-15.27,-29.41,;-16.04,-28.08,;-15.27,-26.76,;-13.73,-26.77,;-12.98,-28.1,;-13.74,-29.42,;-1.29,-29.59,;-1.59,-28.09,)| Show InChI InChI=1S/C28H29N5/c29-18-20-6-11-27-24(17-20)25(19-31-27)21-7-9-22(10-8-21)32-13-15-33(16-14-32)28-5-1-4-26-23(28)3-2-12-30-26/h1-6,11-12,17,19,21-22,31H,7-10,13-16H2/t21-,22+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem 16: 6707-23 (2008)
Article DOI: 10.1016/j.bmc.2008.05.075
BindingDB Entry DOI: 10.7270/Q2GT5MZ4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































