

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine receptor H3 (Macaca mulatta (Rhesus macaque)) | BDBM50262939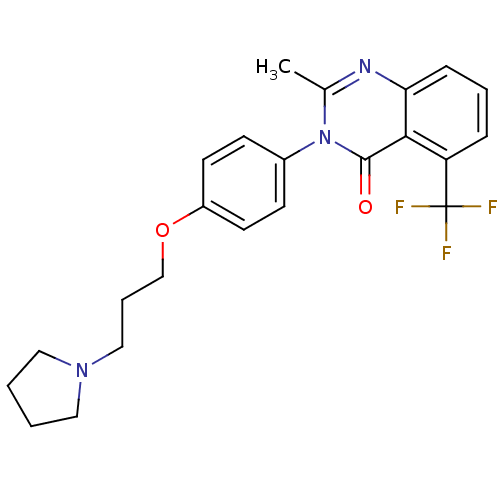 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rhesus monkey histamine H3 receptor expressed in HEK293T cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat histamine H3 receptor expressed in HEK293 cells coexpressed with CRE-beta-lactamase | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262794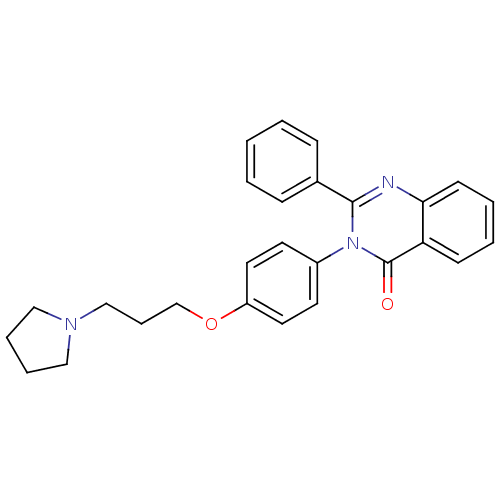 (2-Phenyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262792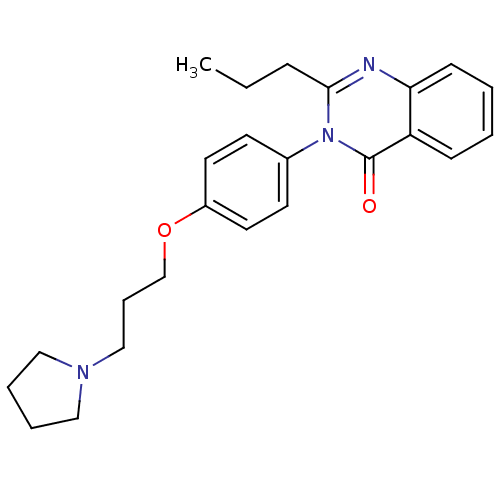 (2-Propyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262747 (2-Methyl-3-[4-({3-[(3S)-3-methyl-1-piperidinyl]pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262793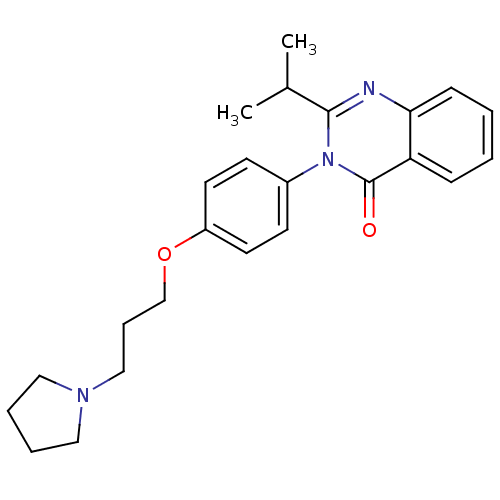 (2-Isopropyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262937 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262795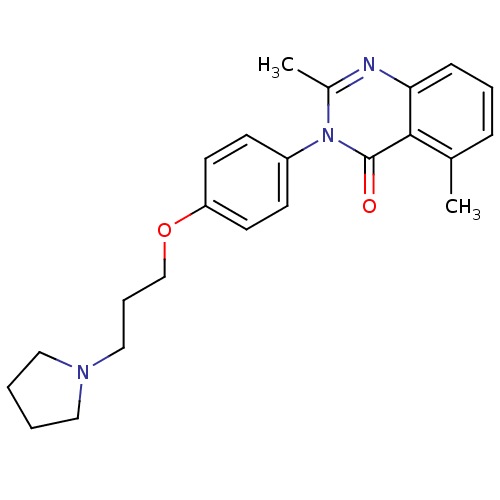 (2,5-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262838 (6-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262835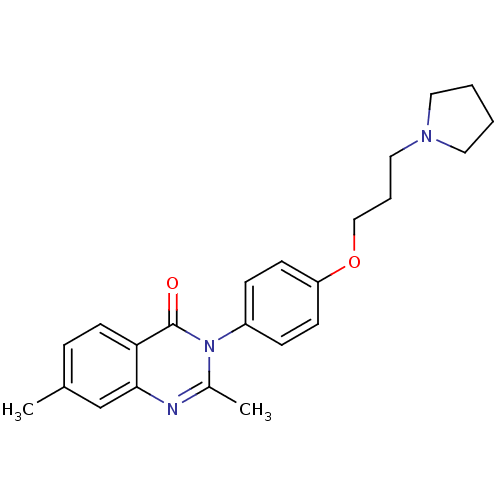 (2,7-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262936 (5-Chloro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262837 (5-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262887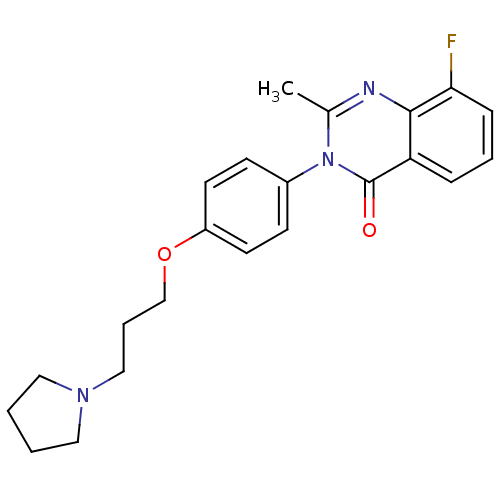 (8-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262791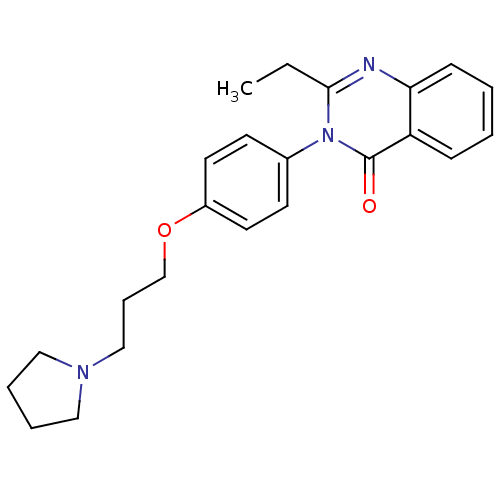 (2-Ethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262885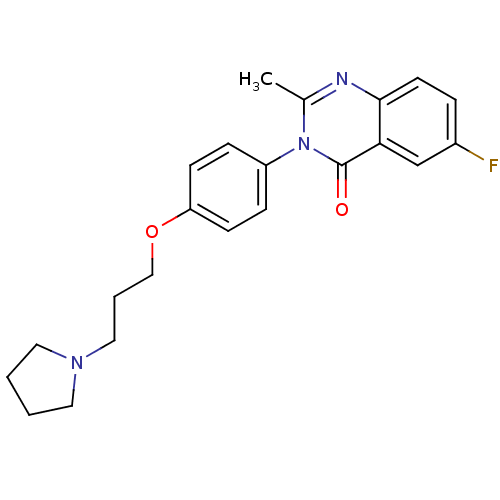 (6-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263294 (3-(4-{[3-(1-Azepanyl)propyl]oxy}phenyl)-2-methyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262836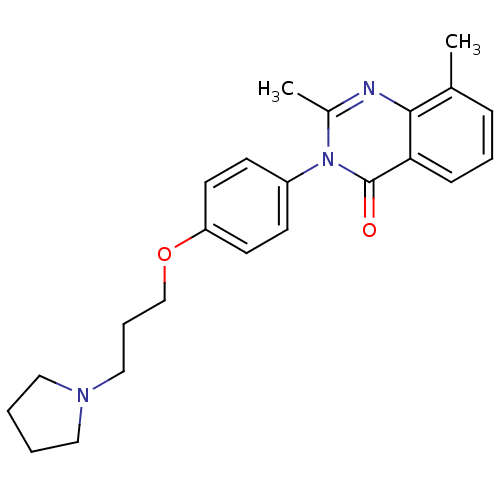 (2,8-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262939 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262886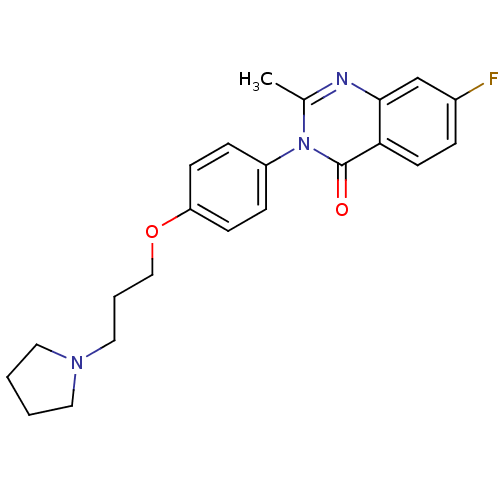 (7-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262884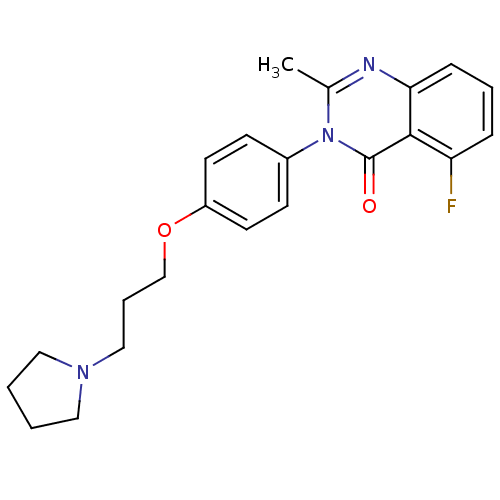 (5-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262938 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262584 (2-Methyl-3-[4-({3-[(2S)-2-methyl-1-pyrrolidinyl]pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263291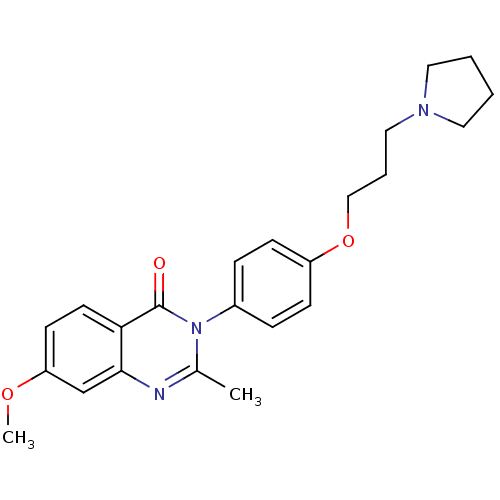 (7-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50246240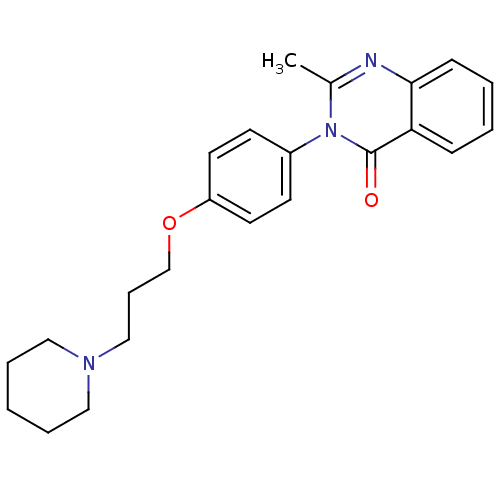 (2-Methyl-3-(4-{[3-(1-piperidinyl)propyl]oxy}phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262751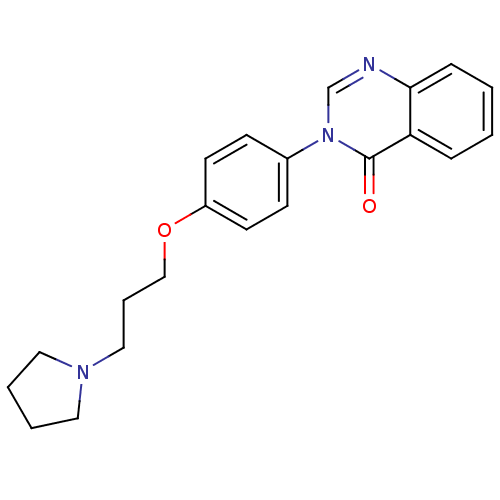 (3-(4-{[3-(1-Pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263293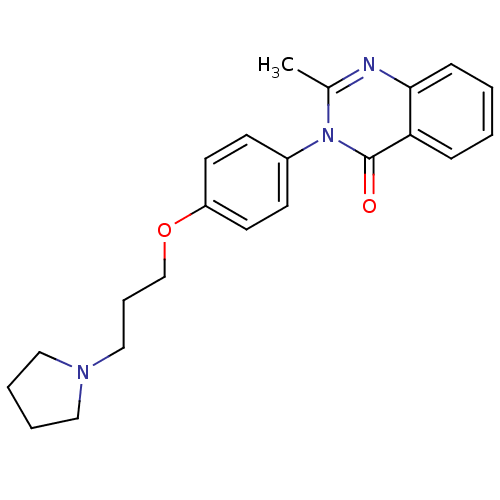 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262883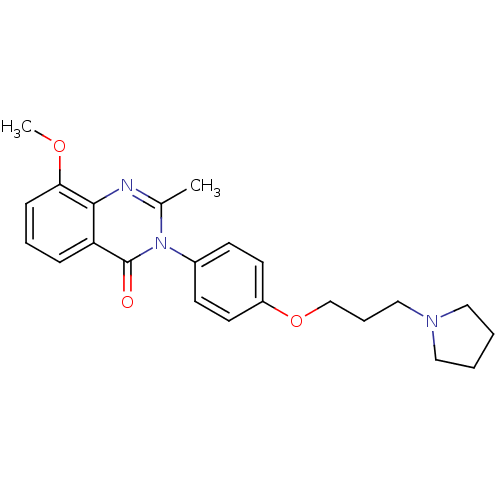 (8-Methoxy-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262834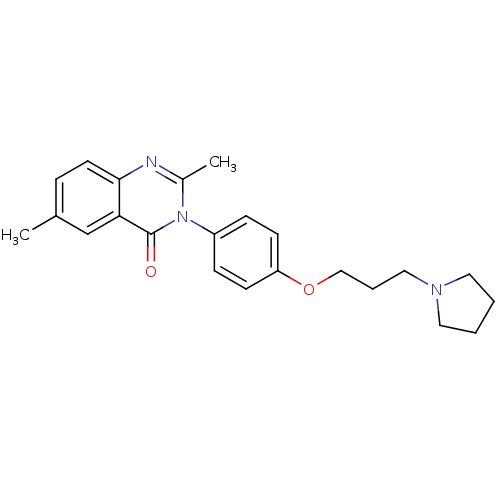 (2,6-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262585 (2-Methyl-3-[4-({3-[(2R)-2-methyl-1-pyrrolidinyl]pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262586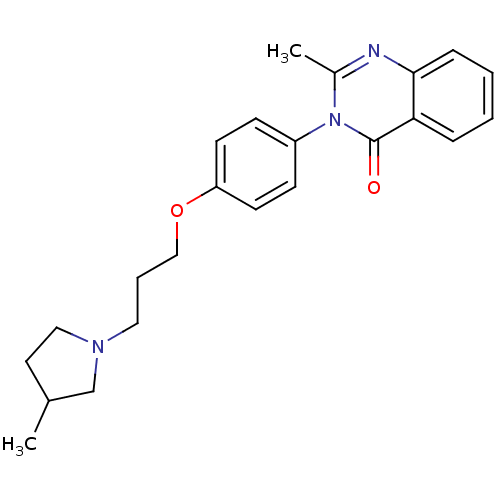 (2-Methyl-3-(4-{[3-(3-methyl-1-pyrrolidinyl)propyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262583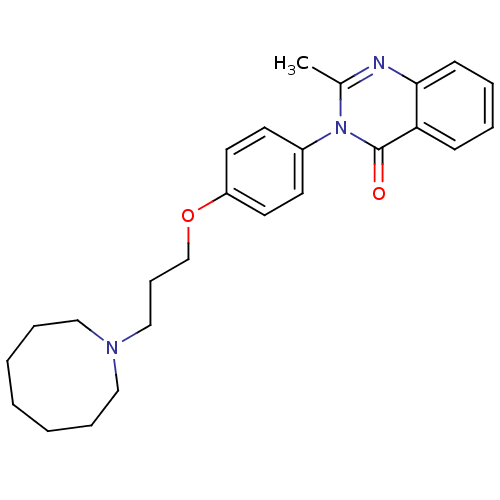 (3-(4-{[3-(1-Azocanyl)propyl]oxy}phenyl)-2-methyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262643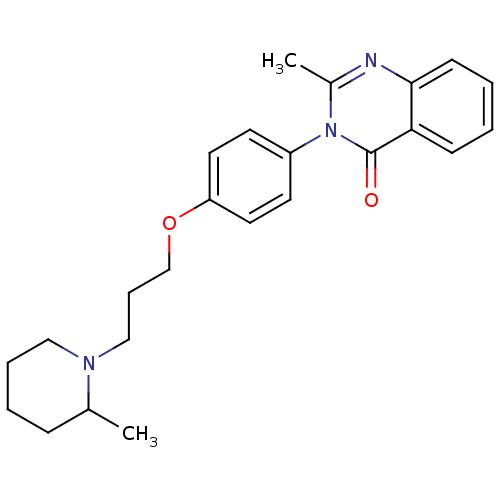 (2-Methyl-3-(4-{[3-(2-methyl-1-piperidinyl)propyl]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262587 (3-[4-({3-[(2R,5R)-2,5-Dimethyl-1-pyrrolidinyl]prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262749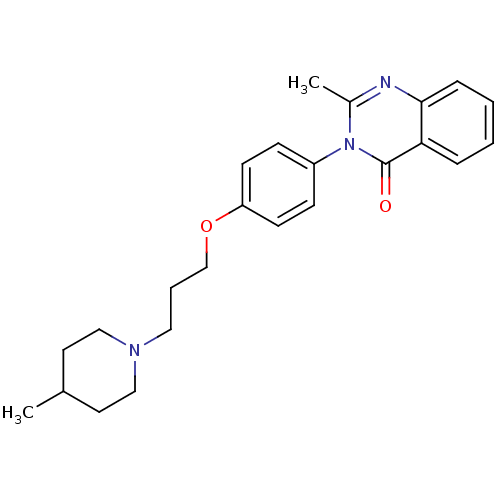 (2-Methyl-3-(4-{[3-(4-methyl-1-piperidinyl)propyl]o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262750 (3-(4-{[3-(3,5-Dimethyl-1-piperidinyl)propyl]oxy}ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263292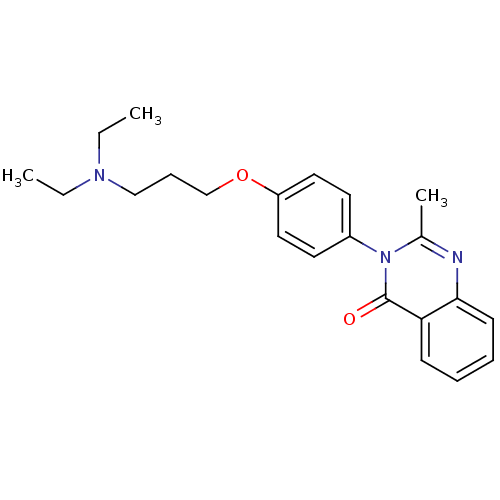 (3-(4-{[3-(Diethylamino)propyl]oxy}phenyl)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50262748 (2-Methyl-3-[4-({3-[(3R)-3-methyl-1-piperidinyl]pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262938 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262751 (3-(4-{[3-(1-Pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262794 (2-Phenyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262836 (2,8-Dimethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262885 (6-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262937 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262792 (2-Propyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262793 (2-Isopropyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262791 (2-Ethyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262747 (2-Methyl-3-[4-({3-[(3S)-3-methyl-1-piperidinyl]pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262584 (2-Methyl-3-[4-({3-[(2S)-2-methyl-1-pyrrolidinyl]pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50262886 (7-Fluoro-2-methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth... | J Med Chem 51: 4780-9 (2008) Article DOI: 10.1021/jm8003834 BindingDB Entry DOI: 10.7270/Q2FQ9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |