 Found 109 hits Enz. Inhib. hit(s) with all data for entry = 50026599
Found 109 hits Enz. Inhib. hit(s) with all data for entry = 50026599 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263229
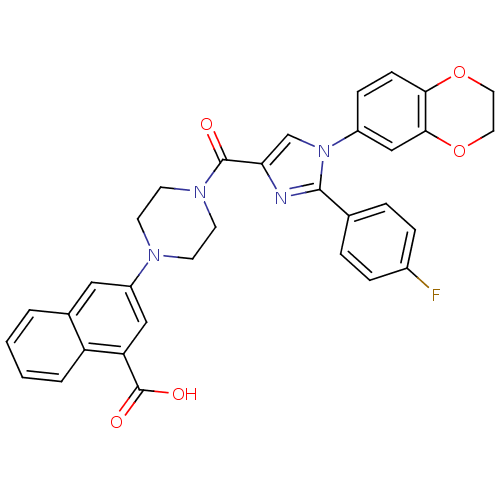
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4...)Show SMILES OC(=O)c1cc(cc2ccccc12)N1CCN(CC1)C(=O)c1cn(c(n1)-c1ccc(F)cc1)-c1ccc2OCCOc2c1 Show InChI InChI=1S/C33H27FN4O5/c34-23-7-5-21(6-8-23)31-35-28(20-38(31)24-9-10-29-30(19-24)43-16-15-42-29)32(39)37-13-11-36(12-14-37)25-17-22-3-1-2-4-26(22)27(18-25)33(40)41/h1-10,17-20H,11-16H2,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263230
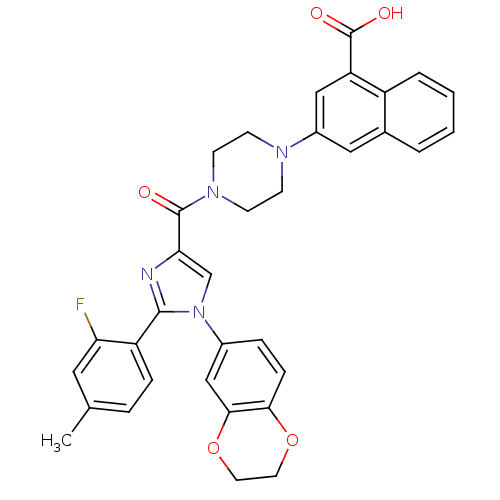
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2...)Show SMILES Cc1ccc(-c2nc(cn2-c2ccc3OCCOc3c2)C(=O)N2CCN(CC2)c2cc(C(O)=O)c3ccccc3c2)c(F)c1 Show InChI InChI=1S/C34H29FN4O5/c1-21-6-8-26(28(35)16-21)32-36-29(20-39(32)23-7-9-30-31(19-23)44-15-14-43-30)33(40)38-12-10-37(11-13-38)24-17-22-4-2-3-5-25(22)27(18-24)34(41)42/h2-9,16-20H,10-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263227

(3-(4-(2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H28F2N4O4/c1-2-43-25-8-5-7-23(18-25)39-20-30(36-31(39)27-11-10-22(34)17-29(27)35)32(40)38-14-12-37(13-15-38)24-16-21-6-3-4-9-26(21)28(19-24)33(41)42/h3-11,16-20H,2,12-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263226
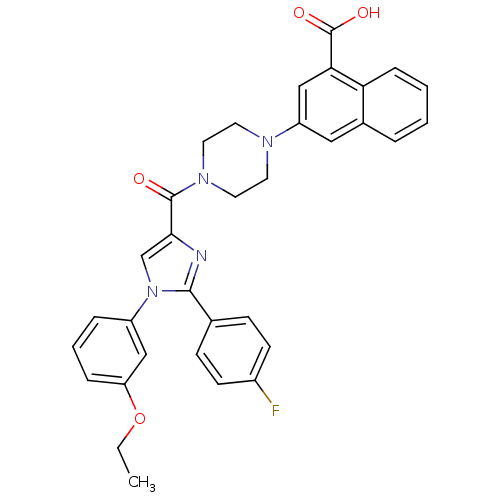
(3-(4-(1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imi...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H29FN4O4/c1-2-42-27-8-5-7-25(19-27)38-21-30(35-31(38)22-10-12-24(34)13-11-22)32(39)37-16-14-36(15-17-37)26-18-23-6-3-4-9-28(23)29(20-26)33(40)41/h3-13,18-21H,2,14-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263225
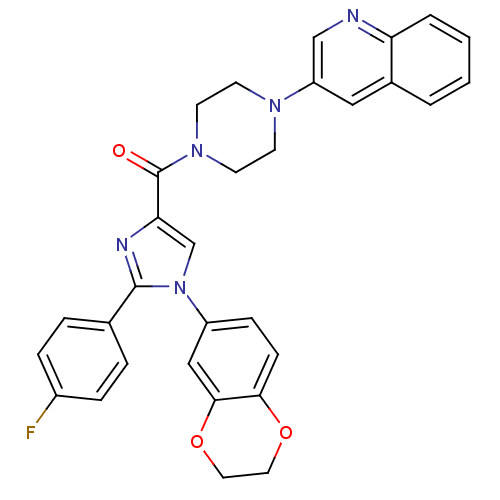
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4-fluo...)Show SMILES Fc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H26FN5O3/c32-23-7-5-21(6-8-23)30-34-27(20-37(30)24-9-10-28-29(18-24)40-16-15-39-28)31(38)36-13-11-35(12-14-36)25-17-22-3-1-2-4-26(22)33-19-25/h1-10,17-20H,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180
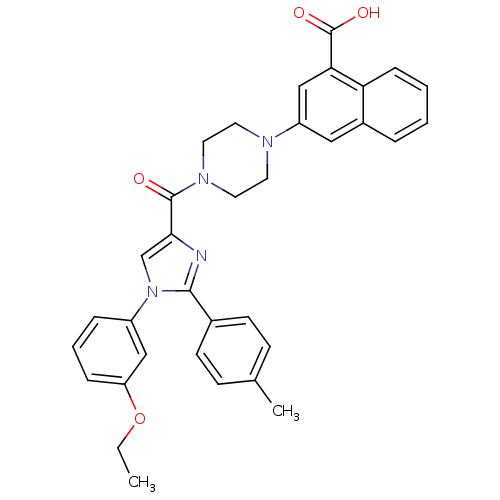
(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262862

(3-(4-(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazole-4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H30N4O4/c1-22-10-12-23(13-11-22)31-34-30(21-37(31)25-7-5-8-27(19-25)41-2)32(38)36-16-14-35(15-17-36)26-18-24-6-3-4-9-28(24)29(20-26)33(39)40/h3-13,18-21H,14-17H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263228
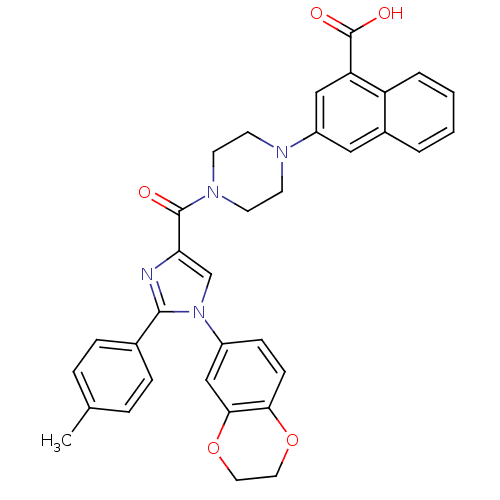
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H30N4O5/c1-22-6-8-23(9-7-22)32-35-29(21-38(32)25-10-11-30-31(20-25)43-17-16-42-30)33(39)37-14-12-36(13-15-37)26-18-24-4-2-3-5-27(24)28(19-26)34(40)41/h2-11,18-21H,12-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263186
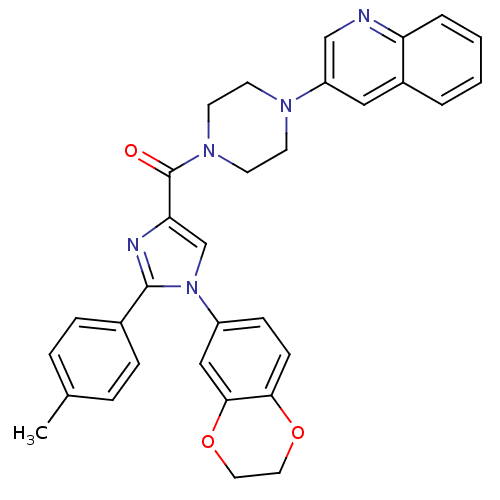
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H29N5O3/c1-22-6-8-23(9-7-22)31-34-28(21-37(31)25-10-11-29-30(19-25)40-17-16-39-29)32(38)36-14-12-35(13-15-36)26-18-24-4-2-3-5-27(24)33-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263185
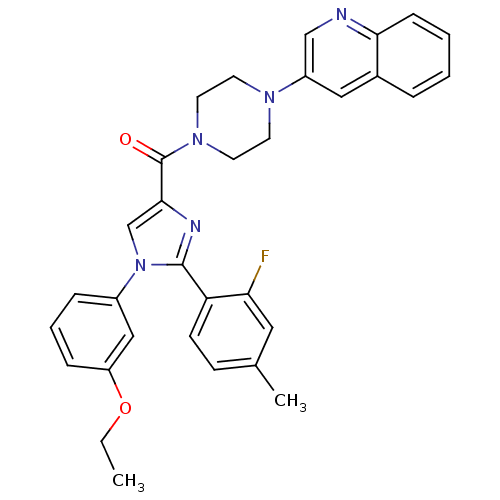
((1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H30FN5O2/c1-3-40-26-9-6-8-24(19-26)38-21-30(35-31(38)27-12-11-22(2)17-28(27)33)32(39)37-15-13-36(14-16-37)25-18-23-7-4-5-10-29(23)34-20-25/h4-12,17-21H,3,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262973
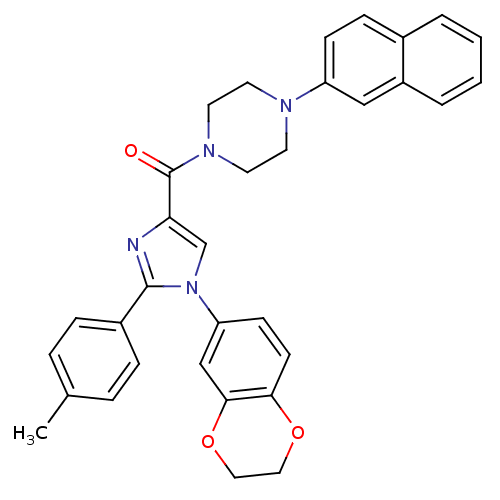
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H30N4O3/c1-23-6-8-25(9-7-23)32-34-29(22-37(32)28-12-13-30-31(21-28)40-19-18-39-30)33(38)36-16-14-35(15-17-36)27-11-10-24-4-2-3-5-26(24)20-27/h2-13,20-22H,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245193
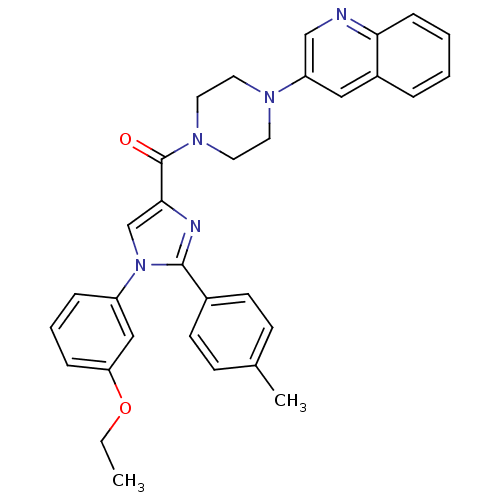
((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H31N5O2/c1-3-39-28-9-6-8-26(20-28)37-22-30(34-31(37)24-13-11-23(2)12-14-24)32(38)36-17-15-35(16-18-36)27-19-25-7-4-5-10-29(25)33-21-27/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263139
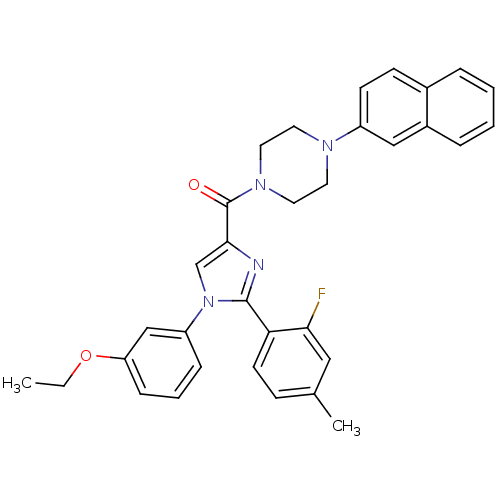
((1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H31FN4O2/c1-3-40-28-10-6-9-27(21-28)38-22-31(35-32(38)29-14-11-23(2)19-30(29)34)33(39)37-17-15-36(16-18-37)26-13-12-24-7-4-5-8-25(24)20-26/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263184
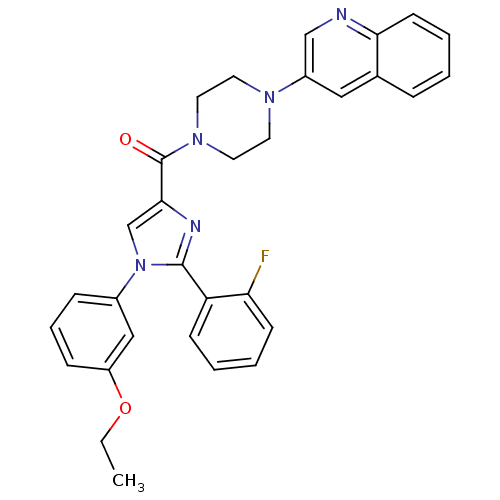
((1-(3-ethoxyphenyl)-2-(2-fluorophenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccccc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H28FN5O2/c1-2-39-25-10-7-9-23(19-25)37-21-29(34-30(37)26-11-4-5-12-27(26)32)31(38)36-16-14-35(15-17-36)24-18-22-8-3-6-13-28(22)33-20-24/h3-13,18-21H,2,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263140
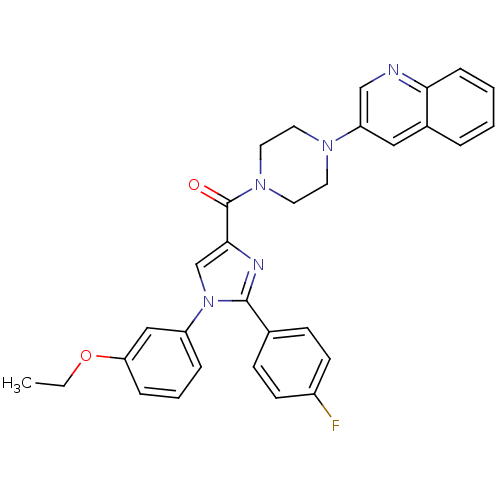
((1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H28FN5O2/c1-2-39-27-8-5-7-25(19-27)37-21-29(34-30(37)22-10-12-24(32)13-11-22)31(38)36-16-14-35(15-17-36)26-18-23-6-3-4-9-28(23)33-20-26/h3-13,18-21H,2,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263104
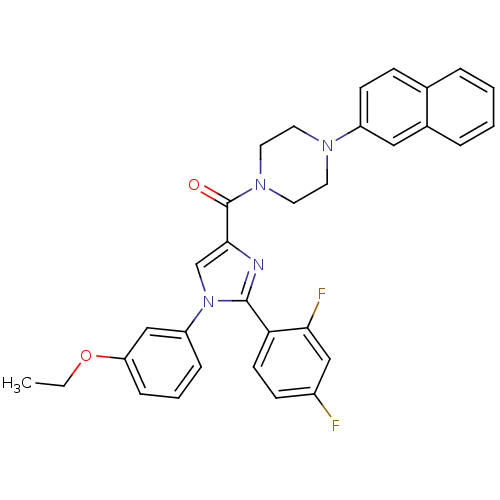
((2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H-imid...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H28F2N4O2/c1-2-40-27-9-5-8-26(20-27)38-21-30(35-31(38)28-13-11-24(33)19-29(28)34)32(39)37-16-14-36(15-17-37)25-12-10-22-6-3-4-7-23(22)18-25/h3-13,18-21H,2,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262817
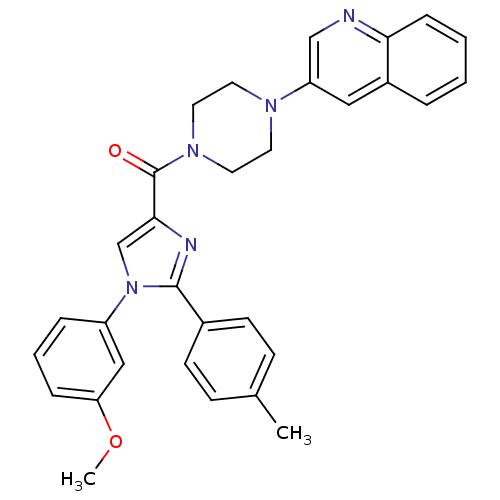
((1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H29N5O2/c1-22-10-12-23(13-11-22)30-33-29(21-36(30)25-7-5-8-27(19-25)38-2)31(37)35-16-14-34(15-17-35)26-18-24-6-3-4-9-28(24)32-20-26/h3-13,18-21H,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262861
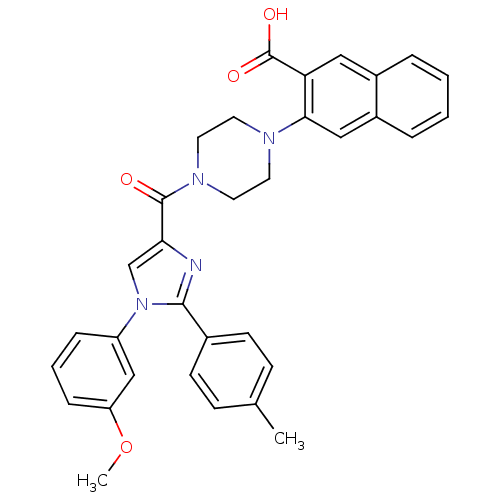
(3-(4-(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazole-4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc2ccccc2cc1C(O)=O Show InChI InChI=1S/C33H30N4O4/c1-22-10-12-23(13-11-22)31-34-29(21-37(31)26-8-5-9-27(20-26)41-2)32(38)36-16-14-35(15-17-36)30-19-25-7-4-3-6-24(25)18-28(30)33(39)40/h3-13,18-21H,14-17H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263103
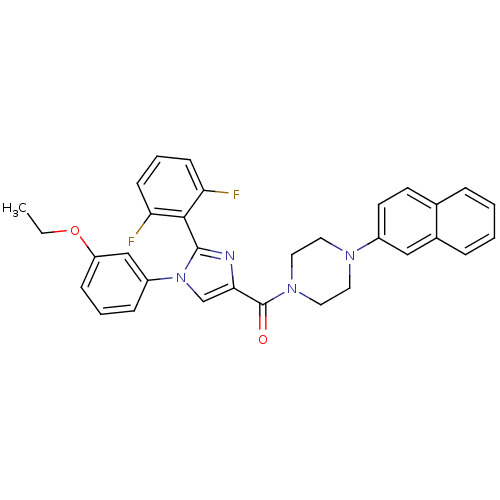
((2-(2,6-difluorophenyl)-1-(3-ethoxyphenyl)-1H-imid...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1c(F)cccc1F)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 |(-.9,-39.94,;-2.01,-41,;-3.49,-40.57,;-4.61,-41.64,;-6.09,-41.2,;-7.2,-42.28,;-6.84,-43.77,;-5.36,-44.19,;-4.24,-43.13,;-5,-45.69,;-3.49,-46.03,;-3.36,-47.57,;-4.77,-48.17,;-5.79,-47.01,;-7.3,-47.27,;-7.84,-48.71,;-6.85,-49.89,;-9.35,-48.96,;-10.34,-47.77,;-9.8,-46.33,;-8.28,-46.07,;-7.74,-44.63,;-2.03,-48.36,;-2.06,-49.9,;-.69,-47.61,;.64,-48.4,;1.99,-47.66,;2.02,-46.11,;.69,-45.32,;-.66,-46.07,;3.37,-45.37,;3.39,-43.84,;4.73,-43.09,;6.06,-43.88,;7.4,-43.15,;8.71,-43.94,;8.69,-45.48,;7.34,-46.22,;6.02,-45.42,;4.68,-46.17,)| Show InChI InChI=1S/C32H28F2N4O2/c1-2-40-26-10-5-9-25(20-26)38-21-29(35-31(38)30-27(33)11-6-12-28(30)34)32(39)37-17-15-36(16-18-37)24-14-13-22-7-3-4-8-23(22)19-24/h3-14,19-21H,2,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263102
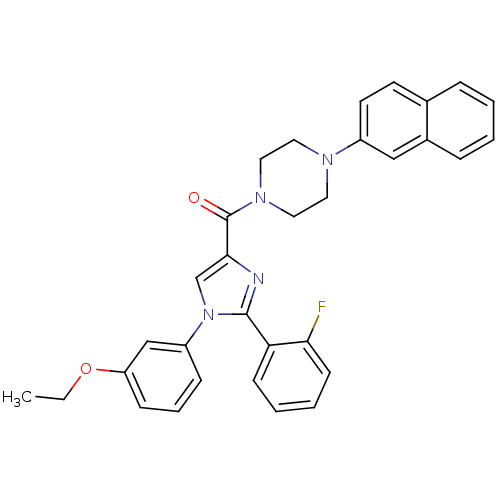
((1-(3-ethoxyphenyl)-2-(2-fluorophenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccccc1F)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H29FN4O2/c1-2-39-27-11-7-10-26(21-27)37-22-30(34-31(37)28-12-5-6-13-29(28)33)32(38)36-18-16-35(17-19-36)25-15-14-23-8-3-4-9-24(23)20-25/h3-15,20-22H,2,16-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262816
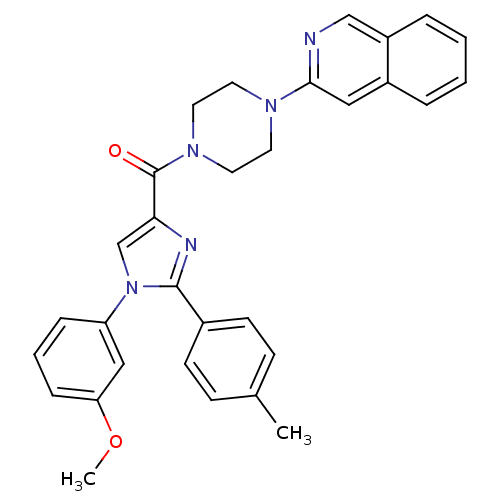
((4-(isoquinolin-3-yl)piperazin-1-yl)(1-(3-methoxyp...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc2ccccc2cn1 Show InChI InChI=1S/C31H29N5O2/c1-22-10-12-23(13-11-22)30-33-28(21-36(30)26-8-5-9-27(19-26)38-2)31(37)35-16-14-34(15-17-35)29-18-24-6-3-4-7-25(24)20-32-29/h3-13,18-21H,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263061
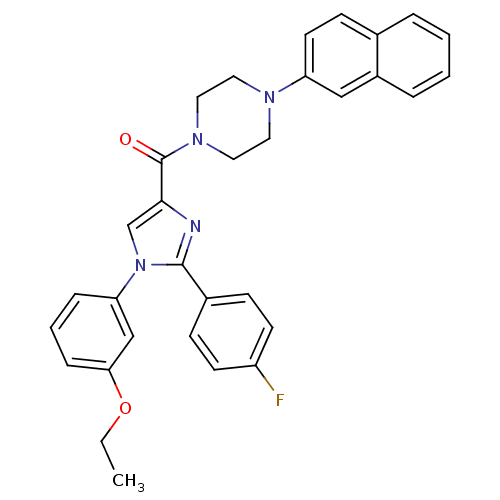
((1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H29FN4O2/c1-2-39-29-9-5-8-28(21-29)37-22-30(34-31(37)24-10-13-26(33)14-11-24)32(38)36-18-16-35(17-19-36)27-15-12-23-6-3-4-7-25(23)20-27/h3-15,20-22H,2,16-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262914
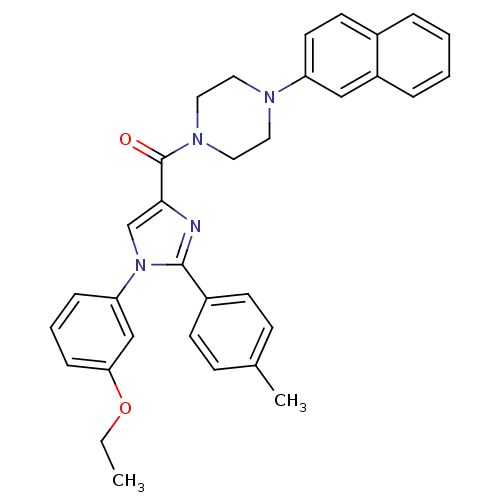
((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H32N4O2/c1-3-39-30-10-6-9-29(22-30)37-23-31(34-32(37)26-13-11-24(2)12-14-26)33(38)36-19-17-35(18-20-36)28-16-15-25-7-4-5-8-27(25)21-28/h4-16,21-23H,3,17-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262776
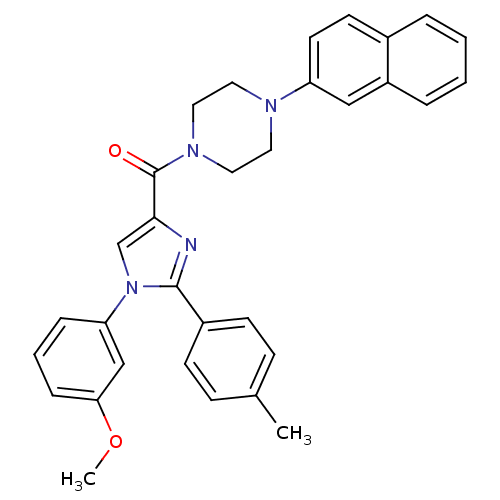
((1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H30N4O2/c1-23-10-12-25(13-11-23)31-33-30(22-36(31)28-8-5-9-29(21-28)38-2)32(37)35-18-16-34(17-19-35)27-15-14-24-6-3-4-7-26(24)20-27/h3-15,20-22H,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263019
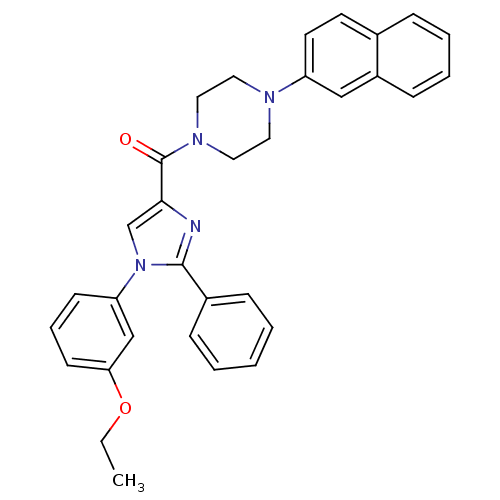
((1-(3-ethoxyphenyl)-2-phenyl-1H-imidazol-4-yl)(4-(...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccccc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H30N4O2/c1-2-38-29-14-8-13-28(22-29)36-23-30(33-31(36)25-10-4-3-5-11-25)32(37)35-19-17-34(18-20-35)27-16-15-24-9-6-7-12-26(24)21-27/h3-16,21-23H,2,17-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263062

((2-(4-chlorophenyl)-1-(3-ethoxyphenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(Cl)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H29ClN4O2/c1-2-39-29-9-5-8-28(21-29)37-22-30(34-31(37)24-10-13-26(33)14-11-24)32(38)36-18-16-35(17-19-36)27-15-12-23-6-3-4-7-25(23)20-27/h3-15,20-22H,2,16-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262863
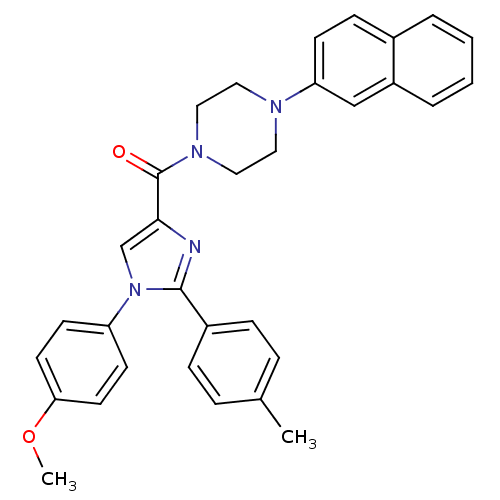
((1-(4-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1ccc(cc1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H30N4O2/c1-23-7-9-25(10-8-23)31-33-30(22-36(31)27-13-15-29(38-2)16-14-27)32(37)35-19-17-34(18-20-35)28-12-11-24-5-3-4-6-26(24)21-28/h3-16,21-22H,17-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262672
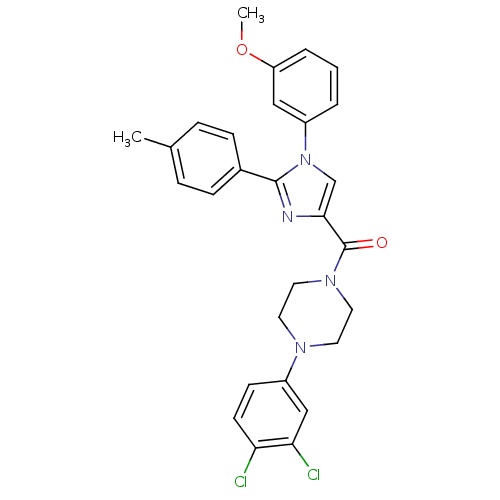
((4-(3,4-dichlorophenyl)piperazin-1-yl)(1-(3-methox...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H26Cl2N4O2/c1-19-6-8-20(9-7-19)27-31-26(18-34(27)22-4-3-5-23(16-22)36-2)28(35)33-14-12-32(13-15-33)21-10-11-24(29)25(30)17-21/h3-11,16-18H,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262859
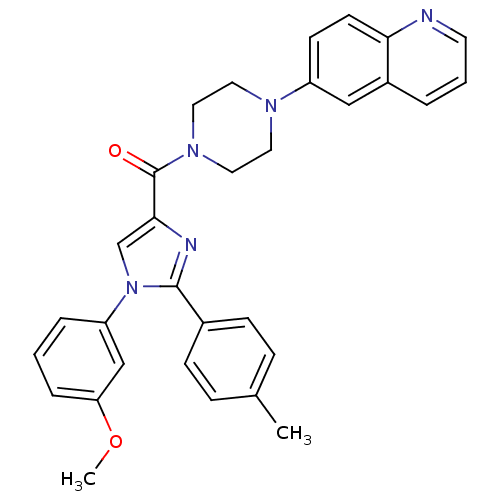
((1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ncccc2c1 Show InChI InChI=1S/C31H29N5O2/c1-22-8-10-23(11-9-22)30-33-29(21-36(30)26-6-3-7-27(20-26)38-2)31(37)35-17-15-34(16-18-35)25-12-13-28-24(19-25)5-4-14-32-28/h3-14,19-21H,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262613

((4-(3-chlorophenyl)piperazin-1-yl)(1-(3-methoxyphe...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H27ClN4O2/c1-20-9-11-21(12-10-20)27-30-26(19-33(27)24-7-4-8-25(18-24)35-2)28(34)32-15-13-31(14-16-32)23-6-3-5-22(29)17-23/h3-12,17-19H,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262815

((1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2n1 Show InChI InChI=1S/C31H29N5O2/c1-22-10-12-24(13-11-22)30-33-28(21-36(30)25-7-5-8-26(20-25)38-2)31(37)35-18-16-34(17-19-35)29-15-14-23-6-3-4-9-27(23)32-29/h3-15,20-21H,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262778
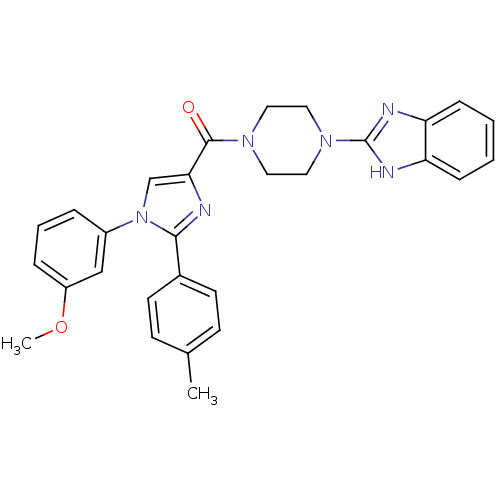
((4-(1H-benzo[d]imidazol-2-yl)piperazin-1-yl)(1-(3-...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C29H28N6O2/c1-20-10-12-21(13-11-20)27-30-26(19-35(27)22-6-5-7-23(18-22)37-2)28(36)33-14-16-34(17-15-33)29-31-24-8-3-4-9-25(24)32-29/h3-13,18-19H,14-17H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262913

((1-(3-hydroxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1cccc(O)c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C31H28N4O2/c1-22-9-11-24(12-10-22)30-32-29(21-35(30)27-7-4-8-28(36)20-27)31(37)34-17-15-33(16-18-34)26-14-13-23-5-2-3-6-25(23)19-26/h2-14,19-21,36H,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262970
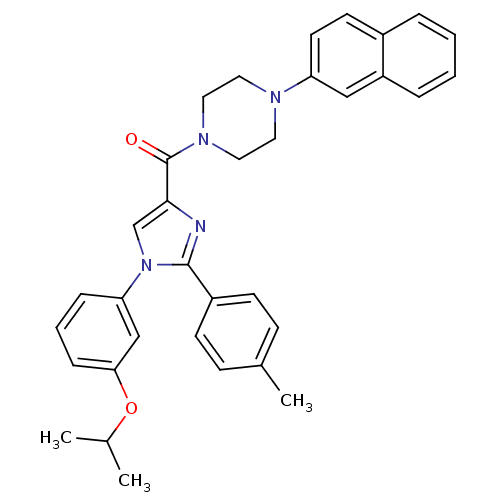
((1-(3-isopropoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl...)Show SMILES CC(C)Oc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C34H34N4O2/c1-24(2)40-31-10-6-9-30(22-31)38-23-32(35-33(38)27-13-11-25(3)12-14-27)34(39)37-19-17-36(18-20-37)29-16-15-26-7-4-5-8-28(26)21-29/h4-16,21-24H,17-20H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263063
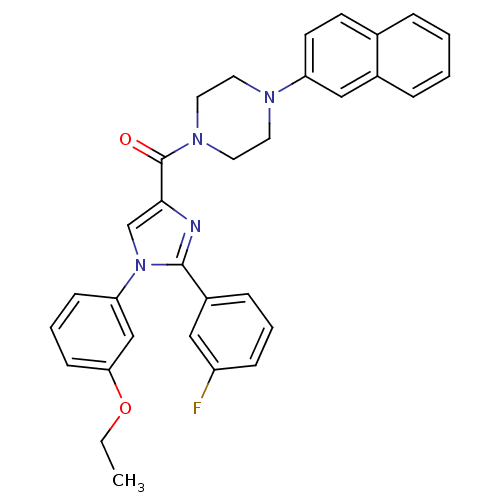
((1-(3-ethoxyphenyl)-2-(3-fluorophenyl)-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1cccc(F)c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H29FN4O2/c1-2-39-29-12-6-11-28(21-29)37-22-30(34-31(37)25-9-5-10-26(33)19-25)32(38)36-17-15-35(16-18-36)27-14-13-23-7-3-4-8-24(23)20-27/h3-14,19-22H,2,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263237
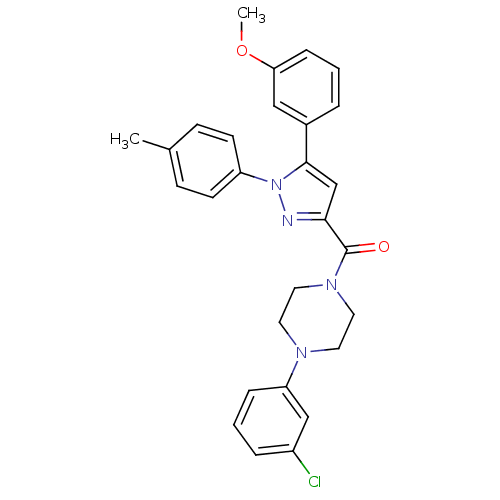
((4-(3-chlorophenyl)piperazin-1-yl)(5-(3-methoxyphe...)Show SMILES COc1cccc(c1)-c1cc(nn1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H27ClN4O2/c1-20-9-11-23(12-10-20)33-27(21-5-3-8-25(17-21)35-2)19-26(30-33)28(34)32-15-13-31(14-16-32)24-7-4-6-22(29)18-24/h3-12,17-19H,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262912
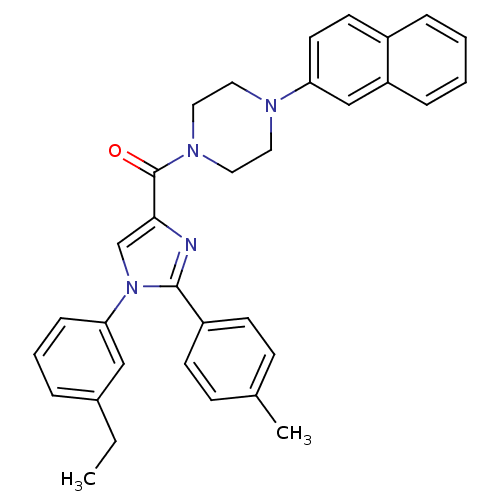
((1-(3-ethylphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-(...)Show SMILES CCc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H32N4O/c1-3-25-7-6-10-30(21-25)37-23-31(34-32(37)27-13-11-24(2)12-14-27)33(38)36-19-17-35(18-20-36)29-16-15-26-8-4-5-9-28(26)22-29/h4-16,21-23H,3,17-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263020
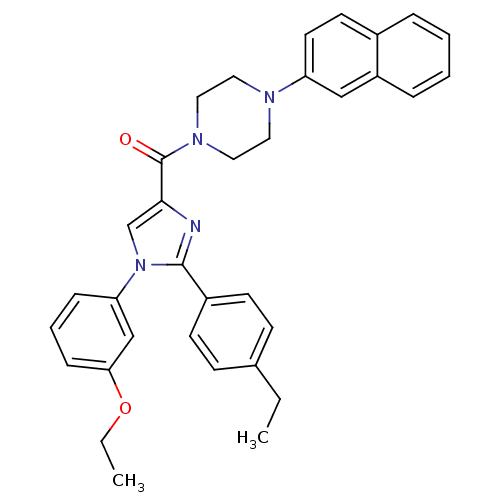
((1-(3-ethoxyphenyl)-2-(4-ethylphenyl)-1H-imidazol-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(CC)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C34H34N4O2/c1-3-25-12-14-27(15-13-25)33-35-32(24-38(33)30-10-7-11-31(23-30)40-4-2)34(39)37-20-18-36(19-21-37)29-17-16-26-8-5-6-9-28(26)22-29/h5-17,22-24H,3-4,18-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262971
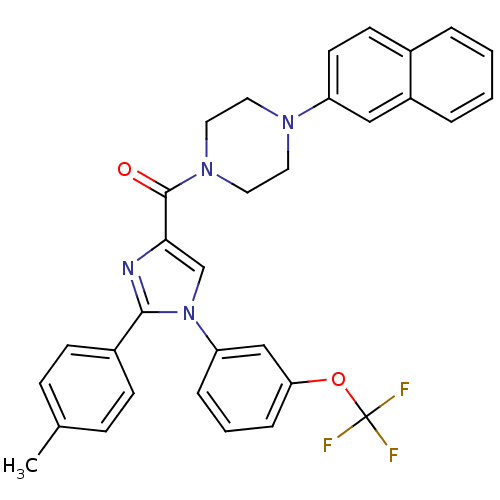
((4-(naphthalen-2-yl)piperazin-1-yl)(2-p-tolyl-1-(3...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1cccc(OC(F)(F)F)c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C32H27F3N4O2/c1-22-9-11-24(12-10-22)30-36-29(21-39(30)27-7-4-8-28(20-27)41-32(33,34)35)31(40)38-17-15-37(16-18-38)26-14-13-23-5-2-3-6-25(23)19-26/h2-14,19-21H,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263018
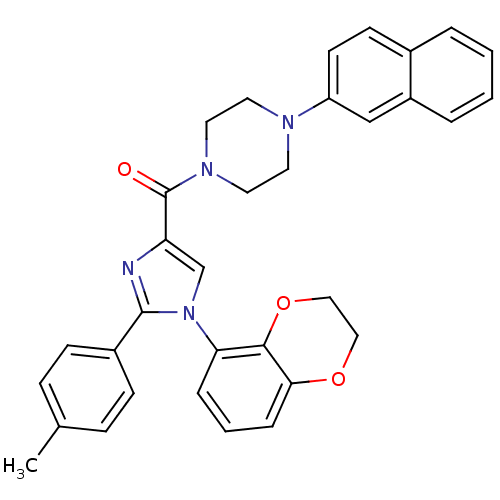
((1-(2,3-dihydrobenzo[b][1,4]dioxin-5-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1cccc2OCCOc12)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H30N4O3/c1-23-9-11-25(12-10-23)32-34-28(22-37(32)29-7-4-8-30-31(29)40-20-19-39-30)33(38)36-17-15-35(16-18-36)27-14-13-24-5-2-3-6-26(24)21-27/h2-14,21-22H,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262969

((4-(naphthalen-2-yl)piperazin-1-yl)(1-(3-propoxyph...)Show SMILES CCCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C34H34N4O2/c1-3-21-40-31-10-6-9-30(23-31)38-24-32(35-33(38)27-13-11-25(2)12-14-27)34(39)37-19-17-36(18-20-37)29-16-15-26-7-4-5-8-28(26)22-29/h4-16,22-24H,3,17-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262860

((4-(isoquinolin-6-yl)piperazin-1-yl)(1-(3-methoxyp...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1ccc2cnccc2c1 Show InChI InChI=1S/C31H29N5O2/c1-22-6-8-23(9-7-22)30-33-29(21-36(30)27-4-3-5-28(19-27)38-2)31(37)35-16-14-34(15-17-35)26-11-10-25-20-32-13-12-24(25)18-26/h3-13,18-21H,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262972

((4-(naphthalen-2-yl)piperazin-1-yl)(2-p-tolyl-1-(3...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1cccc(OCC(F)(F)F)c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H29F3N4O2/c1-23-9-11-25(12-10-23)31-37-30(21-40(31)28-7-4-8-29(20-28)42-22-33(34,35)36)32(41)39-17-15-38(16-18-39)27-14-13-24-5-2-3-6-26(24)19-27/h2-14,19-21H,15-18,22H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262777
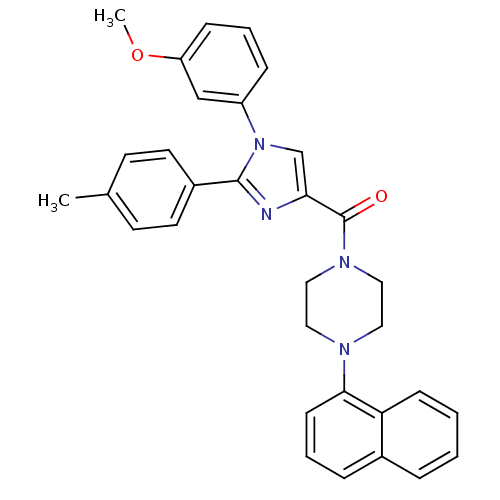
((1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C32H30N4O2/c1-23-13-15-25(16-14-23)31-33-29(22-36(31)26-9-6-10-27(21-26)38-2)32(37)35-19-17-34(18-20-35)30-12-5-8-24-7-3-4-11-28(24)30/h3-16,21-22H,17-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50263229

(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4...)Show SMILES OC(=O)c1cc(cc2ccccc12)N1CCN(CC1)C(=O)c1cn(c(n1)-c1ccc(F)cc1)-c1ccc2OCCOc2c1 Show InChI InChI=1S/C33H27FN4O5/c34-23-7-5-21(6-8-23)31-35-28(20-38(31)24-9-10-29-30(19-24)43-16-15-42-29)32(39)37-13-11-36(12-14-37)25-17-22-3-1-2-4-26(22)27(18-25)33(40)41/h1-10,17-20H,11-16H2,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK2 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50263230

(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2...)Show SMILES Cc1ccc(-c2nc(cn2-c2ccc3OCCOc3c2)C(=O)N2CCN(CC2)c2cc(C(O)=O)c3ccccc3c2)c(F)c1 Show InChI InChI=1S/C34H29FN4O5/c1-21-6-8-26(28(35)16-21)32-36-29(20-39(32)23-7-9-30-31(19-23)44-15-14-43-30)33(40)38-12-10-37(11-13-38)24-17-22-4-2-3-5-25(22)27(18-24)34(41)42/h2-9,16-20H,10-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK2 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50245193

((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H31N5O2/c1-3-39-28-9-6-8-26(20-28)37-22-30(34-31(37)24-13-11-23(2)12-14-24)32(38)36-17-15-35(16-18-36)27-19-25-7-4-5-10-29(25)33-21-27/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK2 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50263228

(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H30N4O5/c1-22-6-8-23(9-7-22)32-35-29(21-38(32)25-10-11-30-31(20-25)43-17-16-42-30)33(39)37-14-12-36(13-15-37)26-18-24-4-2-3-5-27(24)28(19-26)34(40)41/h2-11,18-21H,12-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK2 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50263227

(3-(4-(2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H28F2N4O4/c1-2-43-25-8-5-7-23(18-25)39-20-30(36-31(39)27-11-10-22(34)17-29(27)35)32(40)38-14-12-37(13-15-38)24-16-21-6-3-4-9-26(21)28(19-24)33(41)42/h3-11,16-20H,2,12-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK2 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data