 Found 59 hits Enz. Inhib. hit(s) with all data for entry = 50026855
Found 59 hits Enz. Inhib. hit(s) with all data for entry = 50026855 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245192
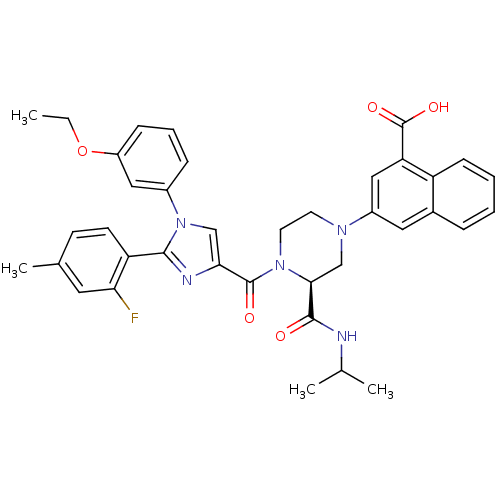
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245186
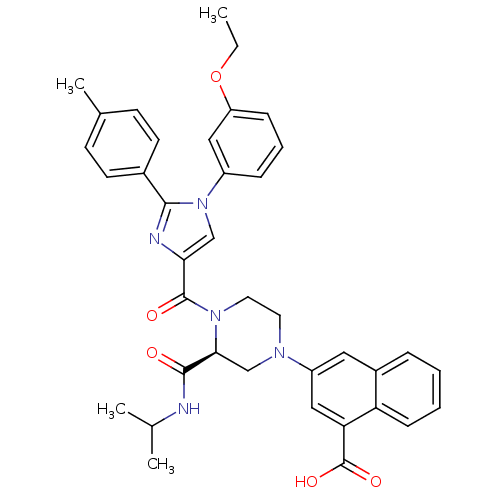
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245205
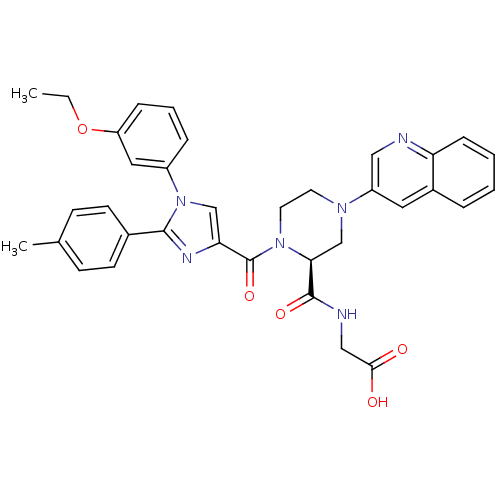
(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245195
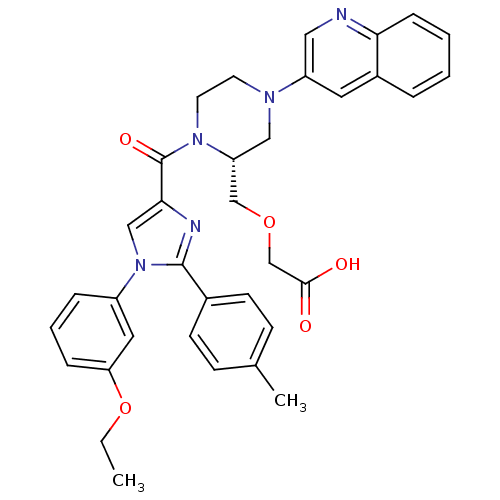
(2-(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245188
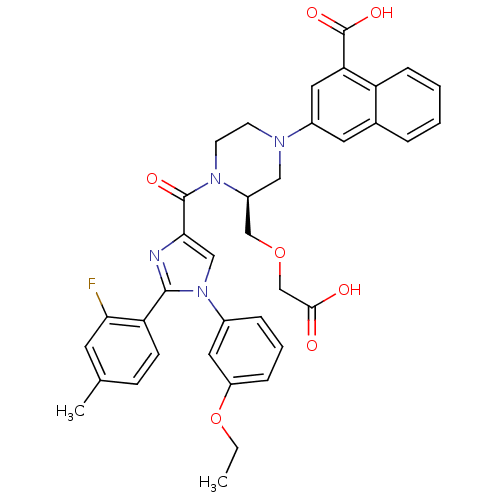
(3-((S)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H35FN4O7/c1-3-49-28-9-6-8-25(17-28)42-20-33(39-35(42)30-12-11-23(2)15-32(30)38)36(45)41-14-13-40(19-27(41)21-48-22-34(43)44)26-16-24-7-4-5-10-29(24)31(18-26)37(46)47/h4-12,15-18,20,27H,3,13-14,19,21-22H2,1-2H3,(H,43,44)(H,46,47)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245201
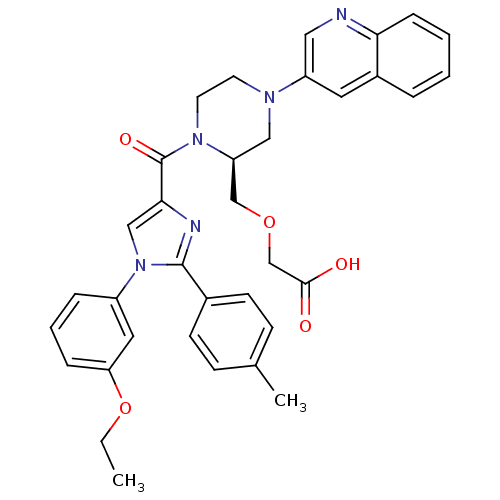
(3-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245189

(3-((R)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36FN5O5/c1-4-48-29-10-7-9-26(18-29)43-22-34(40-35(43)31-13-12-23(2)16-33(31)38)36(45)42-15-14-41(21-28(42)20-39-24(3)44)27-17-25-8-5-6-11-30(25)32(19-27)37(46)47/h5-13,16-19,22,28H,4,14-15,20-21H2,1-3H3,(H,39,44)(H,46,47)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245190
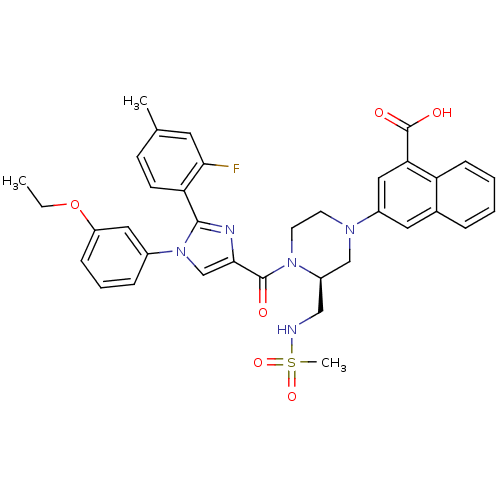
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H36FN5O6S/c1-4-48-28-10-7-9-25(18-28)42-22-33(39-34(42)30-13-12-23(2)16-32(30)37)35(43)41-15-14-40(21-27(41)20-38-49(3,46)47)26-17-24-8-5-6-11-29(24)31(19-26)36(44)45/h5-13,16-19,22,27,38H,4,14-15,20-21H2,1-3H3,(H,44,45)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245182

(3-((S)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37N5O5/c1-4-47-31-10-7-9-28(19-31)42-23-34(39-35(42)26-14-12-24(2)13-15-26)36(44)41-17-16-40(22-30(41)21-38-25(3)43)29-18-27-8-5-6-11-32(27)33(20-29)37(45)46/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,38,43)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245184
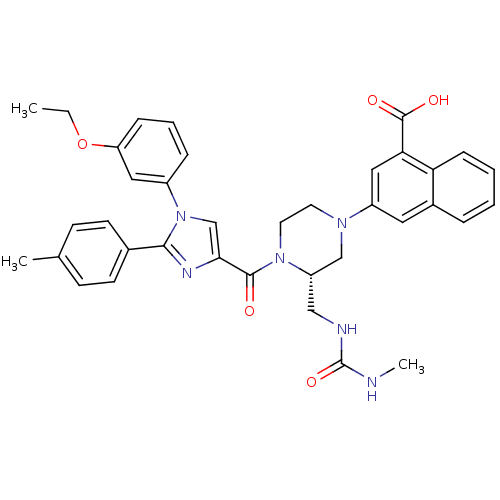
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H38N6O5/c1-4-48-30-10-7-9-27(19-30)43-23-33(40-34(43)25-14-12-24(2)13-15-25)35(44)42-17-16-41(22-29(42)21-39-37(47)38-3)28-18-26-8-5-6-11-31(26)32(20-28)36(45)46/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H,45,46)(H2,38,39,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245191
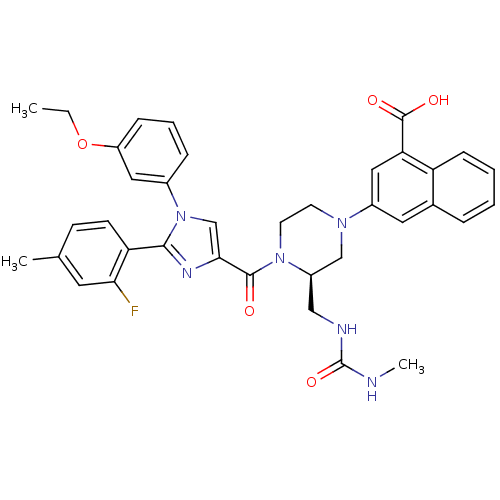
(3-((R)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37FN6O5/c1-4-49-28-10-7-9-25(18-28)44-22-33(41-34(44)30-13-12-23(2)16-32(30)38)35(45)43-15-14-42(21-27(43)20-40-37(48)39-3)26-17-24-8-5-6-11-29(24)31(19-26)36(46)47/h5-13,16-19,22,27H,4,14-15,20-21H2,1-3H3,(H,46,47)(H2,39,40,48)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245185

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245183
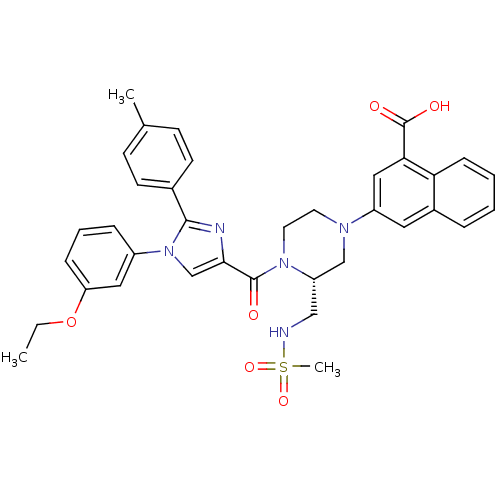
(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H37N5O6S/c1-4-47-30-10-7-9-27(19-30)41-23-33(38-34(41)25-14-12-24(2)13-15-25)35(42)40-17-16-39(22-29(40)21-37-48(3,45)46)28-18-26-8-5-6-11-31(26)32(20-28)36(43)44/h5-15,18-20,23,29,37H,4,16-17,21-22H2,1-3H3,(H,43,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180
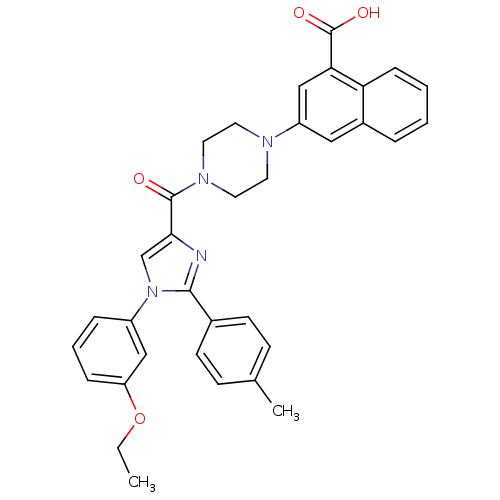
(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245181
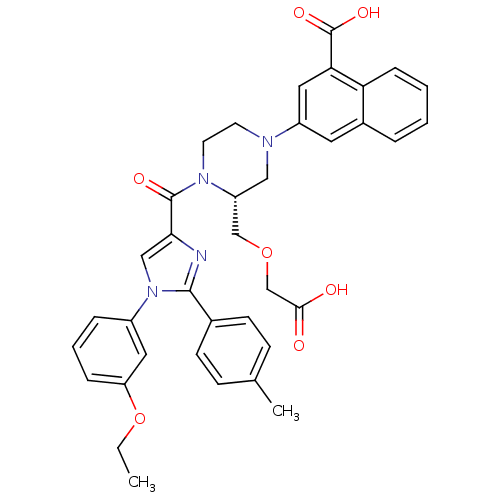
(3-((R)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36N4O7/c1-3-48-30-9-6-8-27(18-30)41-21-33(38-35(41)25-13-11-24(2)12-14-25)36(44)40-16-15-39(20-29(40)22-47-23-34(42)43)28-17-26-7-4-5-10-31(26)32(19-28)37(45)46/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,42,43)(H,45,46)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245199
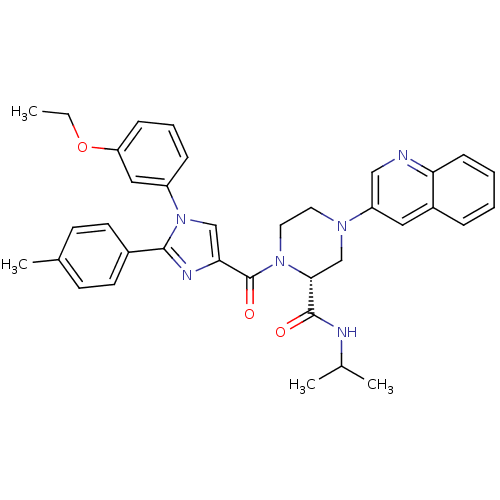
((2R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245204

((2S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245194
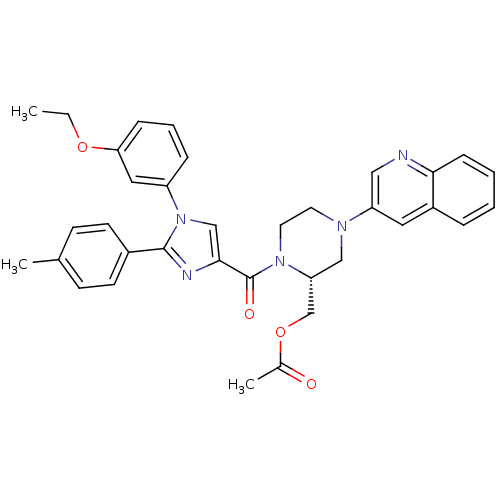
(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245196
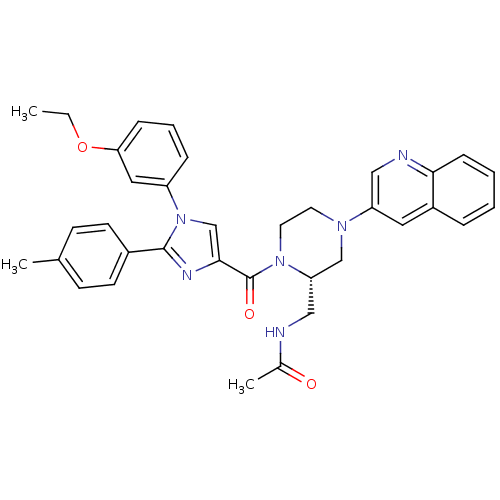
(CHEMBL450443 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245202
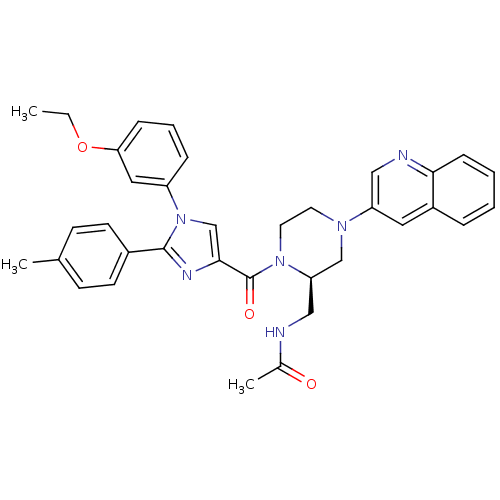
(CHEMBL503331 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245197
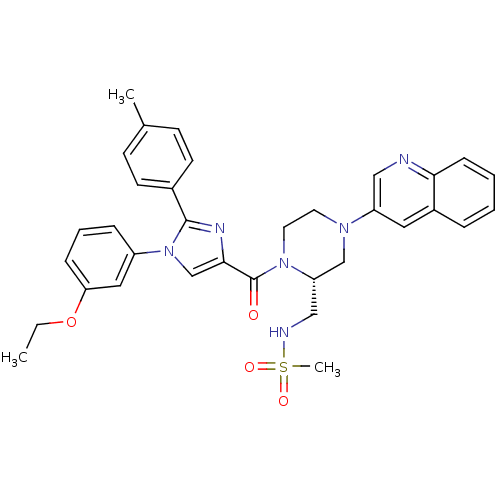
(CHEMBL449725 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C34H36N6O4S/c1-4-44-30-10-7-9-27(19-30)40-23-32(37-33(40)25-14-12-24(2)13-15-25)34(41)39-17-16-38(22-29(39)21-36-45(3,42)43)28-18-26-8-5-6-11-31(26)35-20-28/h5-15,18-20,23,29,36H,4,16-17,21-22H2,1-3H3/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245193
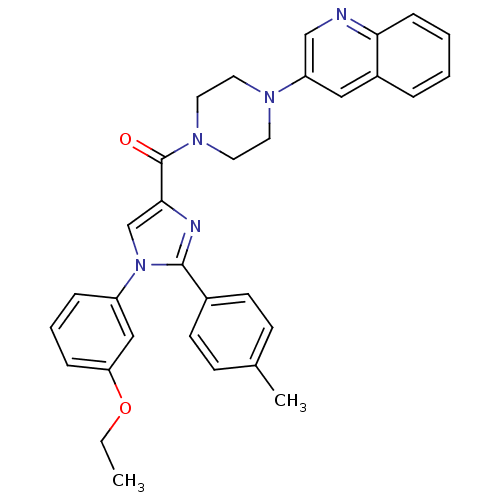
((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H31N5O2/c1-3-39-28-9-6-8-26(20-28)37-22-30(34-31(37)24-13-11-23(2)12-14-24)32(38)36-17-15-35(16-18-36)27-19-25-7-4-5-10-29(25)33-21-27/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245200
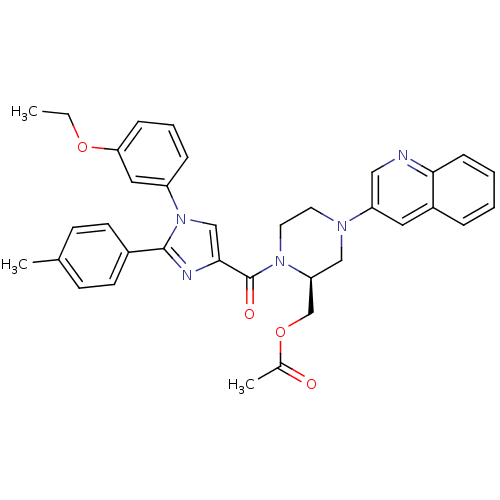
(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245198
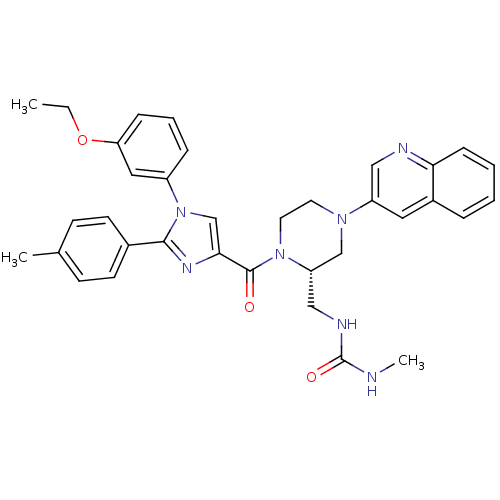
(1-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H37N7O3/c1-4-45-30-10-7-9-27(19-30)42-23-32(39-33(42)25-14-12-24(2)13-15-25)34(43)41-17-16-40(22-29(41)21-38-35(44)36-3)28-18-26-8-5-6-11-31(26)37-20-28/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H2,36,38,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245203

(CHEMBL448118 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C34H36N6O4S/c1-4-44-30-10-7-9-27(19-30)40-23-32(37-33(40)25-14-12-24(2)13-15-25)34(41)39-17-16-38(22-29(39)21-36-45(3,42)43)28-18-26-8-5-6-11-31(26)35-20-28/h5-15,18-20,23,29,36H,4,16-17,21-22H2,1-3H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245186

(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245185

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245184

(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H38N6O5/c1-4-48-30-10-7-9-27(19-30)43-23-33(40-34(43)25-14-12-24(2)13-15-25)35(44)42-17-16-41(22-29(42)21-39-37(47)38-3)28-18-26-8-5-6-11-31(26)32(20-28)36(45)46/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H,45,46)(H2,38,39,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245183

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H37N5O6S/c1-4-47-30-10-7-9-27(19-30)41-23-33(38-34(41)25-14-12-24(2)13-15-25)35(42)40-17-16-39(22-29(40)21-37-48(3,45)46)28-18-26-8-5-6-11-31(26)32(20-28)36(43)44/h5-15,18-20,23,29,37H,4,16-17,21-22H2,1-3H3,(H,43,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245182

(3-((S)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37N5O5/c1-4-47-31-10-7-9-28(19-31)42-23-34(39-35(42)26-14-12-24(2)13-15-26)36(44)41-17-16-40(22-30(41)21-38-25(3)43)29-18-27-8-5-6-11-32(27)33(20-29)37(45)46/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,38,43)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245188

(3-((S)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H35FN4O7/c1-3-49-28-9-6-8-25(17-28)42-20-33(39-35(42)30-12-11-23(2)15-32(30)38)36(45)41-14-13-40(19-27(41)21-48-22-34(43)44)26-16-24-7-4-5-10-29(24)31(18-26)37(46)47/h4-12,15-18,20,27H,3,13-14,19,21-22H2,1-2H3,(H,43,44)(H,46,47)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245189

(3-((R)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36FN5O5/c1-4-48-29-10-7-9-26(18-29)43-22-34(40-35(43)31-13-12-23(2)16-33(31)38)36(45)42-15-14-41(21-28(42)20-39-24(3)44)27-17-25-8-5-6-11-30(25)32(19-27)37(46)47/h5-13,16-19,22,28H,4,14-15,20-21H2,1-3H3,(H,39,44)(H,46,47)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245190

(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H36FN5O6S/c1-4-48-28-10-7-9-25(18-28)42-22-33(39-34(42)30-13-12-23(2)16-32(30)37)35(43)41-15-14-40(21-27(41)20-38-49(3,46)47)26-17-24-8-5-6-11-29(24)31(19-26)36(44)45/h5-13,16-19,22,27,38H,4,14-15,20-21H2,1-3H3,(H,44,45)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245191

(3-((R)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37FN6O5/c1-4-49-28-10-7-9-25(18-28)44-22-33(41-34(44)30-13-12-23(2)16-32(30)38)35(45)43-15-14-42(21-27(43)20-40-37(48)39-3)26-17-24-8-5-6-11-29(24)31(19-26)36(46)47/h5-13,16-19,22,27H,4,14-15,20-21H2,1-3H3,(H,46,47)(H2,39,40,48)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245192

(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245193

((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H31N5O2/c1-3-39-28-9-6-8-26(20-28)37-22-30(34-31(37)24-13-11-23(2)12-14-24)32(38)36-17-15-35(16-18-36)27-19-25-7-4-5-10-29(25)33-21-27/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245194

(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245195

(2-(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245196

(CHEMBL450443 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245197

(CHEMBL449725 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C34H36N6O4S/c1-4-44-30-10-7-9-27(19-30)40-23-32(37-33(40)25-14-12-24(2)13-15-25)34(41)39-17-16-38(22-29(39)21-36-45(3,42)43)28-18-26-8-5-6-11-31(26)35-20-28/h5-15,18-20,23,29,36H,4,16-17,21-22H2,1-3H3/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245198

(1-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H37N7O3/c1-4-45-30-10-7-9-27(19-30)42-23-32(39-33(42)25-14-12-24(2)13-15-25)34(43)41-17-16-40(22-29(41)21-38-35(44)36-3)28-18-26-8-5-6-11-31(26)37-20-28/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H2,36,38,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245199

((2R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245200

(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245201

(3-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245202

(CHEMBL503331 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245203

(CHEMBL448118 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C34H36N6O4S/c1-4-44-30-10-7-9-27(19-30)40-23-32(37-33(40)25-14-12-24(2)13-15-25)34(41)39-17-16-38(22-29(39)21-36-45(3,42)43)28-18-26-8-5-6-11-31(26)35-20-28/h5-15,18-20,23,29,36H,4,16-17,21-22H2,1-3H3/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245204

((2S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245205

(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data