 Found 54 hits Enz. Inhib. hit(s) with all data for entry = 50046708
Found 54 hits Enz. Inhib. hit(s) with all data for entry = 50046708 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127174
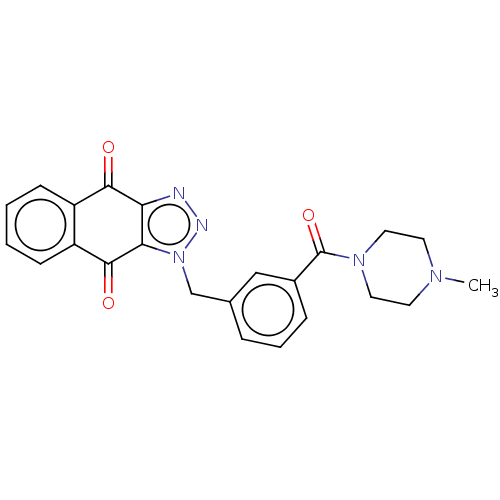
(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127173
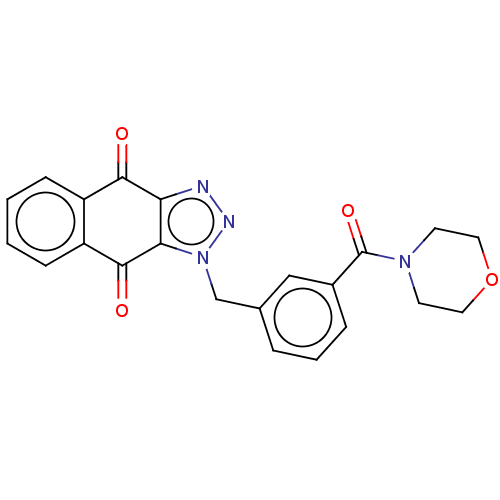
(CHEMBL3628598)Show SMILES O=C(N1CCOCC1)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C22H18N4O4/c27-20-16-6-1-2-7-17(16)21(28)19-18(20)23-24-26(19)13-14-4-3-5-15(12-14)22(29)25-8-10-30-11-9-25/h1-7,12H,8-11,13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127164
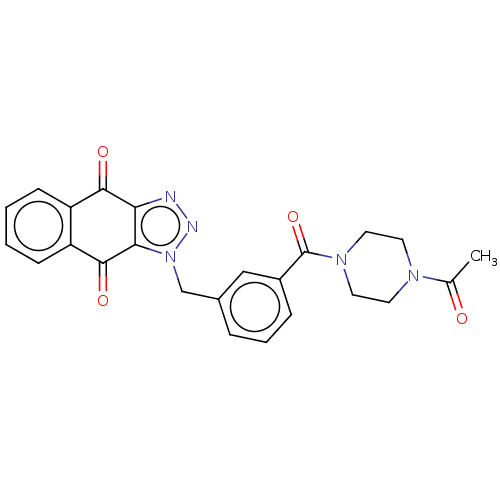
(CHEMBL3628597)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C24H21N5O4/c1-15(30)27-9-11-28(12-10-27)24(33)17-6-4-5-16(13-17)14-29-21-20(25-26-29)22(31)18-7-2-3-8-19(18)23(21)32/h2-8,13H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127160

(CHEMBL3628595)Show SMILES CCN(CC)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C22H20N4O3/c1-3-25(4-2)22(29)15-9-7-8-14(12-15)13-26-19-18(23-24-26)20(27)16-10-5-6-11-17(16)21(19)28/h5-12H,3-4,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127204
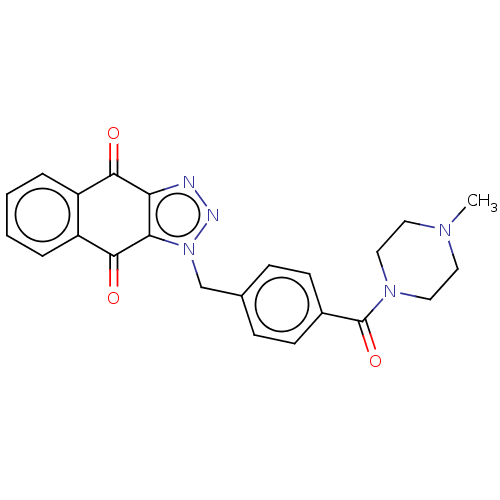
(CHEMBL3628602)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C23H21N5O3/c1-26-10-12-27(13-11-26)23(31)16-8-6-15(7-9-16)14-28-20-19(24-25-28)21(29)17-4-2-3-5-18(17)22(20)30/h2-9H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127203

(CHEMBL3628601)Show SMILES O=C(N1CCOCC1)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C22H18N4O4/c27-20-16-3-1-2-4-17(16)21(28)19-18(20)23-24-26(19)13-14-5-7-15(8-6-14)22(29)25-9-11-30-12-10-25/h1-8H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127206
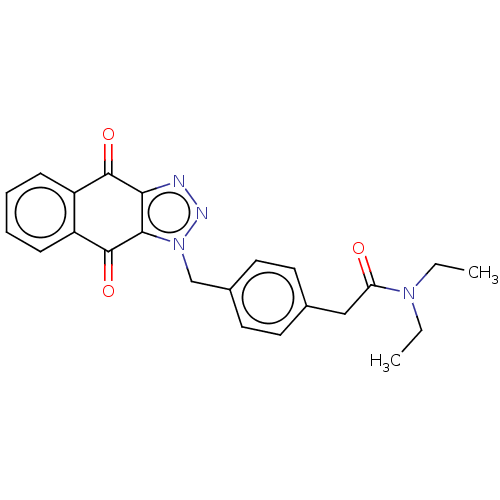
(CHEMBL3628604)Show SMILES CCN(CC)C(=O)Cc1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C23H22N4O3/c1-3-26(4-2)19(28)13-15-9-11-16(12-10-15)14-27-21-20(24-25-27)22(29)17-7-5-6-8-18(17)23(21)30/h5-12H,3-4,13-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127158
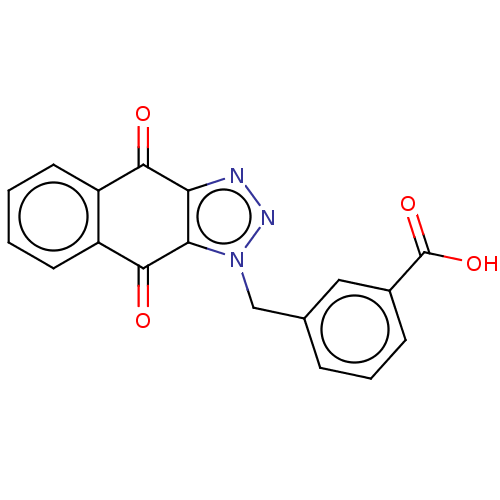
(CHEMBL3628593)Show InChI InChI=1S/C18H11N3O4/c22-16-12-6-1-2-7-13(12)17(23)15-14(16)19-20-21(15)9-10-4-3-5-11(8-10)18(24)25/h1-8H,9H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127175
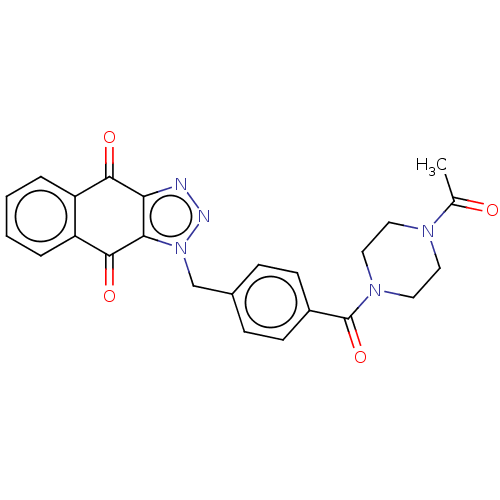
(CHEMBL3628600)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C24H21N5O4/c1-15(30)27-10-12-28(13-11-27)24(33)17-8-6-16(7-9-17)14-29-21-20(25-26-29)22(31)18-4-2-3-5-19(18)23(21)32/h2-9H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127161
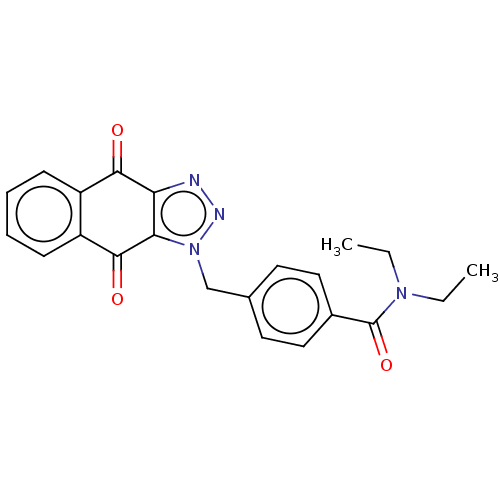
(CHEMBL3628596)Show SMILES CCN(CC)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C22H20N4O3/c1-3-25(4-2)22(29)15-11-9-14(10-12-15)13-26-19-18(23-24-26)20(27)16-7-5-6-8-17(16)21(19)28/h5-12H,3-4,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127205
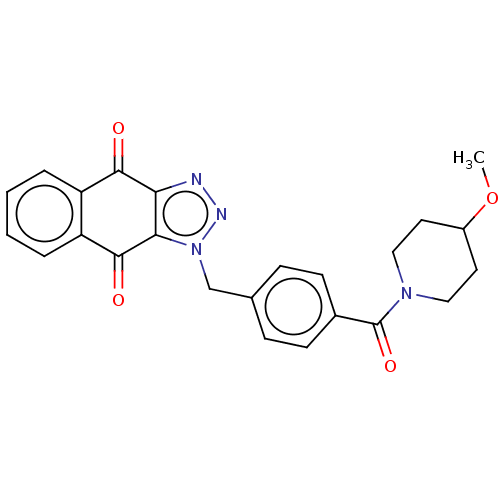
(CHEMBL3628603)Show SMILES COC1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C24H22N4O4/c1-32-17-10-12-27(13-11-17)24(31)16-8-6-15(7-9-16)14-28-21-20(25-26-28)22(29)18-4-2-3-5-19(18)23(21)30/h2-9,17H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127159

(CHEMBL3628594)Show InChI InChI=1S/C18H11N3O4/c22-16-12-3-1-2-4-13(12)17(23)15-14(16)19-20-21(15)9-10-5-7-11(8-6-10)18(24)25/h1-8H,9H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127136
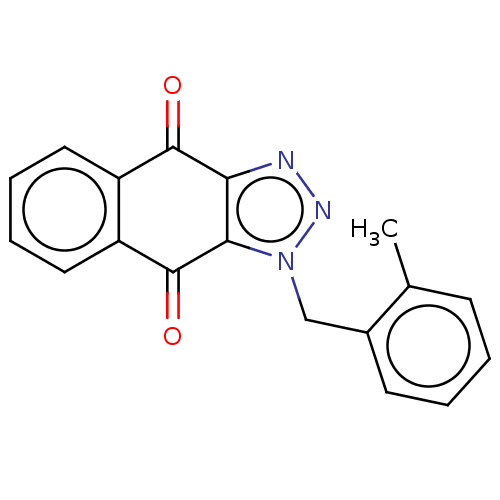
(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 561 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127164

(CHEMBL3628597)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C24H21N5O4/c1-15(30)27-9-11-28(12-10-27)24(33)17-6-4-5-16(13-17)14-29-21-20(25-26-29)22(31)18-7-2-3-8-19(18)23(21)32/h2-8,13H,9-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 602 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127135
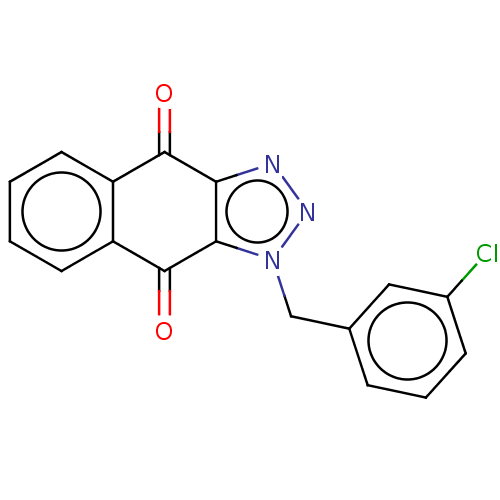
(CHEMBL3628551)Show InChI InChI=1S/C17H10ClN3O2/c18-11-5-3-4-10(8-11)9-21-15-14(19-20-21)16(22)12-6-1-2-7-13(12)17(15)23/h1-8H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 711 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127135

(CHEMBL3628551)Show InChI InChI=1S/C17H10ClN3O2/c18-11-5-3-4-10(8-11)9-21-15-14(19-20-21)16(22)12-6-1-2-7-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 767 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127155
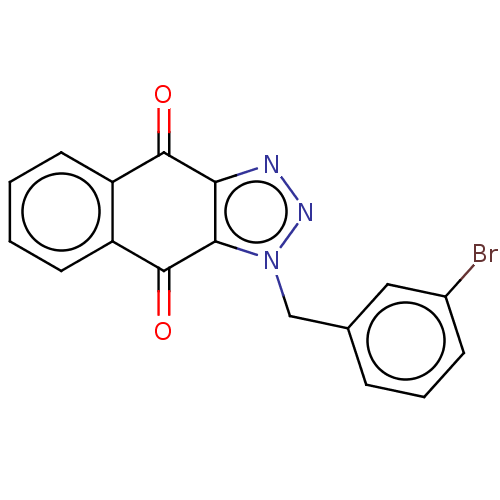
(CHEMBL3628554)Show InChI InChI=1S/C17H10BrN3O2/c18-11-5-3-4-10(8-11)9-21-15-14(19-20-21)16(22)12-6-1-2-7-13(12)17(15)23/h1-8H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 768 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127136

(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 809 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127206

(CHEMBL3628604)Show SMILES CCN(CC)C(=O)Cc1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C23H22N4O3/c1-3-26(4-2)19(28)13-15-9-11-16(12-10-15)14-27-21-20(24-25-27)22(29)17-7-5-6-8-18(17)23(21)30/h5-12H,3-4,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 922 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127173

(CHEMBL3628598)Show SMILES O=C(N1CCOCC1)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C22H18N4O4/c27-20-16-6-1-2-7-17(16)21(28)19-18(20)23-24-26(19)13-14-4-3-5-15(12-14)22(29)25-8-10-30-11-9-25/h1-7,12H,8-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 936 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127160

(CHEMBL3628595)Show SMILES CCN(CC)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C22H20N4O3/c1-3-25(4-2)22(29)15-9-7-8-14(12-15)13-26-19-18(23-24-26)20(27)16-10-5-6-11-17(16)21(19)28/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 977 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127203

(CHEMBL3628601)Show SMILES O=C(N1CCOCC1)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C22H18N4O4/c27-20-16-3-1-2-4-17(16)21(28)19-18(20)23-24-26(19)13-14-5-7-15(8-6-14)22(29)25-9-11-30-12-10-25/h1-8H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 982 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127139
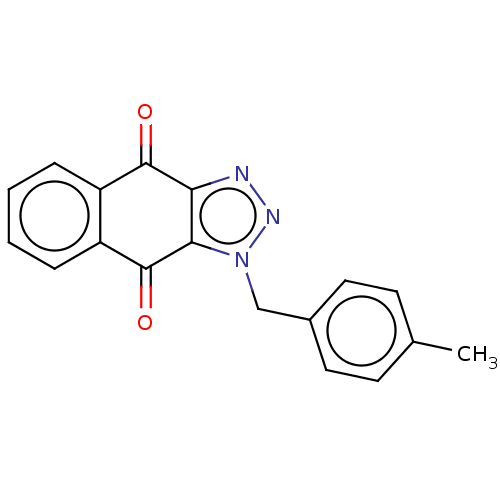
(CHEMBL3628553)Show InChI InChI=1S/C18H13N3O2/c1-11-6-8-12(9-7-11)10-21-16-15(19-20-21)17(22)13-4-2-3-5-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127175

(CHEMBL3628600)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C24H21N5O4/c1-15(30)27-10-12-28(13-11-27)24(33)17-8-6-16(7-9-17)14-29-21-20(25-26-29)22(31)18-4-2-3-5-19(18)23(21)32/h2-9H,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127158

(CHEMBL3628593)Show InChI InChI=1S/C18H11N3O4/c22-16-12-6-1-2-7-13(12)17(23)15-14(16)19-20-21(15)9-10-4-3-5-11(8-10)18(24)25/h1-8H,9H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127156
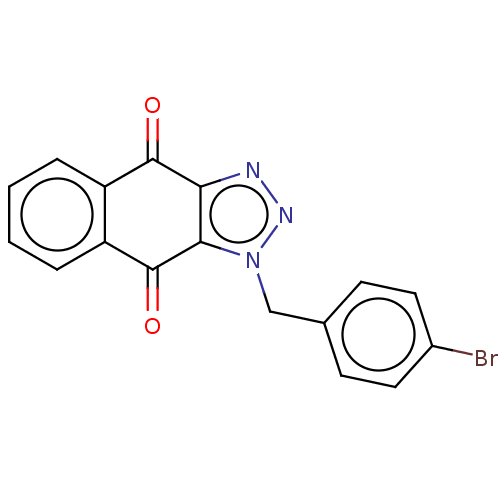
(CHEMBL3628555)Show InChI InChI=1S/C17H10BrN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127205

(CHEMBL3628603)Show SMILES COC1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C24H22N4O4/c1-32-17-10-12-27(13-11-17)24(31)16-8-6-15(7-9-16)14-28-21-20(25-26-28)22(29)18-4-2-3-5-19(18)23(21)30/h2-9,17H,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM70489
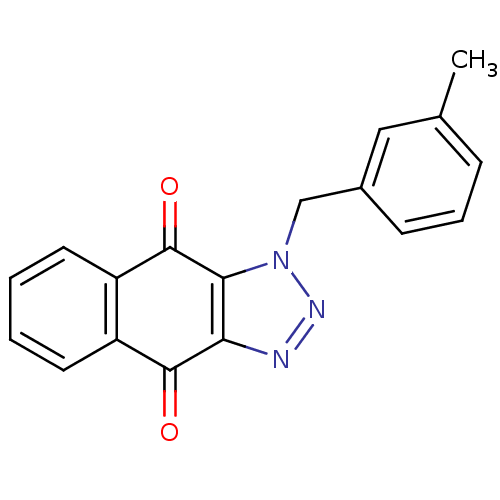
(1-(3-methylbenzyl)-1H-naphtho[2,3-d][1,2,3]triazol...)Show InChI InChI=1S/C18H13N3O2/c1-11-5-4-6-12(9-11)10-21-16-15(19-20-21)17(22)13-7-2-3-8-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127139

(CHEMBL3628553)Show InChI InChI=1S/C18H13N3O2/c1-11-6-8-12(9-7-11)10-21-16-15(19-20-21)17(22)13-4-2-3-5-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127161

(CHEMBL3628596)Show SMILES CCN(CC)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C22H20N4O3/c1-3-25(4-2)22(29)15-11-9-14(10-12-15)13-26-19-18(23-24-26)20(27)16-7-5-6-8-17(16)21(19)28/h5-12H,3-4,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127204

(CHEMBL3628602)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)cc1 Show InChI InChI=1S/C23H21N5O3/c1-26-10-12-27(13-11-26)23(31)16-8-6-15(7-9-16)14-28-20-19(24-25-28)21(29)17-4-2-3-5-18(17)22(20)30/h2-9H,10-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127159

(CHEMBL3628594)Show InChI InChI=1S/C18H11N3O4/c22-16-12-3-1-2-4-13(12)17(23)15-14(16)19-20-21(15)9-10-5-7-11(8-6-10)18(24)25/h1-8H,9H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127137
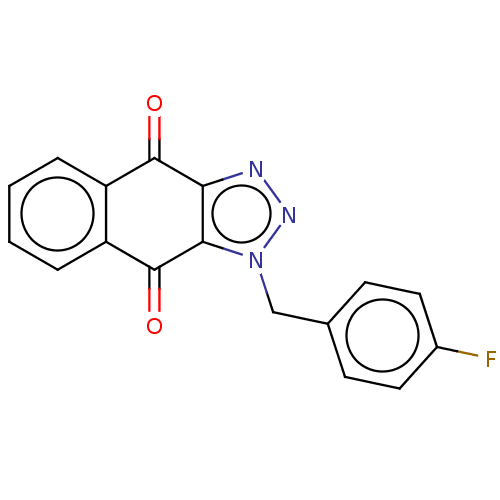
(CHEMBL3628552)Show InChI InChI=1S/C17H10FN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127155

(CHEMBL3628554)Show InChI InChI=1S/C17H10BrN3O2/c18-11-5-3-4-10(8-11)9-21-15-14(19-20-21)16(22)12-6-1-2-7-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM70489

(1-(3-methylbenzyl)-1H-naphtho[2,3-d][1,2,3]triazol...)Show InChI InChI=1S/C18H13N3O2/c1-11-5-4-6-12(9-11)10-21-16-15(19-20-21)17(22)13-7-2-3-8-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127157
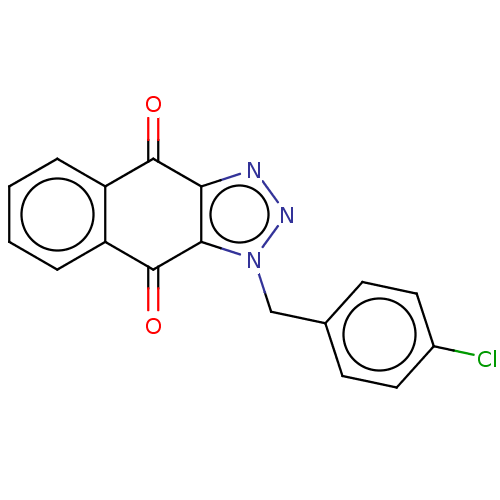
(CHEMBL3628556)Show InChI InChI=1S/C17H10ClN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50127137

(CHEMBL3628552)Show InChI InChI=1S/C17H10FN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50127136

(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Displacement of 125I-SDF-1 from human CXCR4 expressed in HEK293 cells incubated for 1.5 hrs by Topcount analysis |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) expressed in Sf9 insect cells for 4 hrs by Kinase-Glo assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50127136

(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) expressed in Sf9 insect cells for 4 hrs by Kinase-Glo assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mu-opioid receptor (unknown origin) expressed in CHO cells for 60 mins |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50127136

(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mu-opioid receptor (unknown origin) expressed in CHO cells for 60 mins |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR incubated for 2 hrs by Kinase-Glo luminescent kinase assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50127136

(CHEMBL1442185)Show InChI InChI=1S/C18H13N3O2/c1-11-6-2-3-7-12(11)10-21-16-15(19-20-21)17(22)13-8-4-5-9-14(13)18(16)23/h2-9H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human EGFR incubated for 2 hrs by Kinase-Glo luminescent kinase assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B preincubated with protein for 15 mins followed by DiFMUP addition for 1 hr measured by fluorescence analysis |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50127135

(CHEMBL3628551)Show InChI InChI=1S/C17H10ClN3O2/c18-11-5-3-4-10(8-11)9-21-15-14(19-20-21)16(22)12-6-1-2-7-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B preincubated with protein for 15 mins followed by DiFMUP addition for 1 hr measured by fluorescence analysis |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127157

(CHEMBL3628556)Show InChI InChI=1S/C17H10ClN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50127156

(CHEMBL3628555)Show InChI InChI=1S/C17H10BrN3O2/c18-11-7-5-10(6-8-11)9-21-15-14(19-20-21)16(22)12-3-1-2-4-13(12)17(15)23/h1-8H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |
Catalase
(Homo sapiens (Human)) | BDBM50127174

(CHEMBL3628599)Show SMILES CN1CCN(CC1)C(=O)c1cccc(Cn2nnc3c2C(=O)c2ccccc2C3=O)c1 Show InChI InChI=1S/C23H21N5O3/c1-26-9-11-27(12-10-26)23(31)16-6-4-5-15(13-16)14-28-20-19(24-25-28)21(29)17-7-2-3-8-18(17)22(20)30/h2-8,13H,9-12,14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of catalase (unknown origin) |
J Med Chem 58: 7807-19 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00921
BindingDB Entry DOI: 10.7270/Q2JS9S7K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data