 Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50027361
Found 41 hits Enz. Inhib. hit(s) with all data for entry = 50027361 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274028

(CHEMBL499477 | N-(3,5-dichloropyridin-4-yl)-1-(3-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H22Cl2N6O5S/c1-18-37-32(45-39-18)24(20-8-10-22(11-9-20)46(2,43)44)14-19-5-3-6-21(13-19)40-17-25(29(41)23-7-4-12-36-30(23)40)31(42)38-28-26(33)15-35-16-27(28)34/h3-17H,1-2H3,(H,35,38,42)/b24-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274031
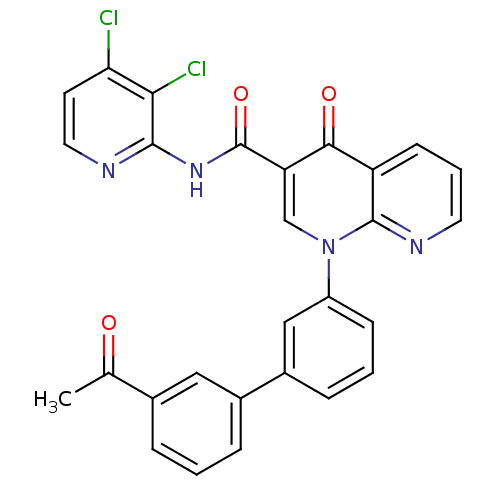
(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(=O)c1cccc(c1)-c1cccc(c1)-n1cc(C(=O)Nc2nccc(Cl)c2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C28H18Cl2N4O3/c1-16(35)17-5-2-6-18(13-17)19-7-3-8-20(14-19)34-15-22(25(36)21-9-4-11-32-27(21)34)28(37)33-26-24(30)23(29)10-12-31-26/h2-15H,1H3,(H,31,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274188
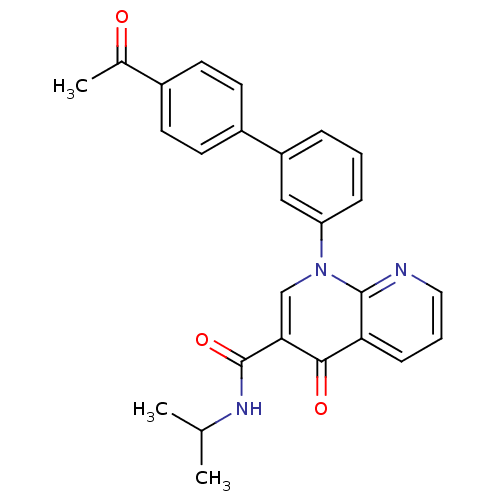
(1-(4'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2ccc(cc2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)8-5-13-27-25)21-7-4-6-20(14-21)19-11-9-18(10-12-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274789

(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2cccc(c2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)11-6-12-27-25)21-10-5-9-20(14-21)19-8-4-7-18(13-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274027

(1-(4-chlorophenyl)-N-(3,5-dichloropyridin-4-yl)-4-...)Show SMILES Clc1ccc(cc1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C20H11Cl3N4O2/c21-11-3-5-12(6-4-11)27-10-14(18(28)13-2-1-7-25-19(13)27)20(29)26-17-15(22)8-24-9-16(17)23/h1-10H,(H,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274189
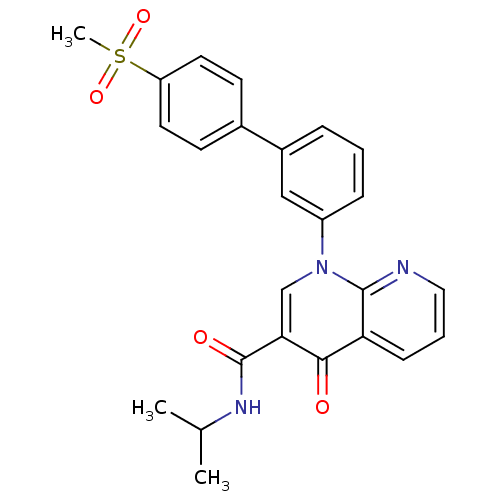
(1-(4'-Methanesulfonyl-biphenyl-3-yl)-4-oxo-1,4-dih...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2ccc(cc2)S(C)(=O)=O)c2ncccc2c1=O Show InChI InChI=1S/C25H23N3O4S/c1-16(2)27-25(30)22-15-28(24-21(23(22)29)8-5-13-26-24)19-7-4-6-18(14-19)17-9-11-20(12-10-17)33(3,31)32/h4-16H,1-3H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274063
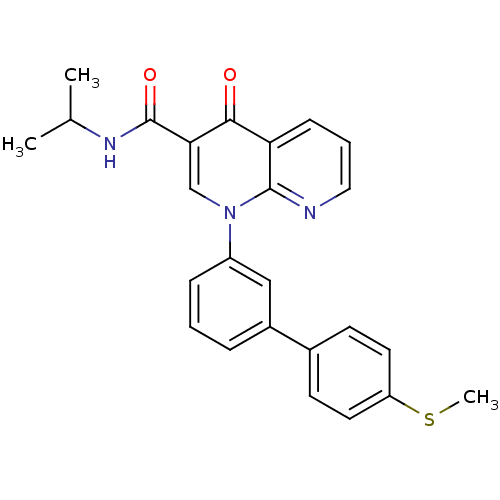
(1-(4'-Methylsulfanyl-biphenyl-3-yl)-4-oxo-1,4-dihy...)Show SMILES CSc1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NC(C)C)c(=O)c2cccnc12 Show InChI InChI=1S/C25H23N3O2S/c1-16(2)27-25(30)22-15-28(24-21(23(22)29)8-5-13-26-24)19-7-4-6-18(14-19)17-9-11-20(31-3)12-10-17/h4-16H,1-3H3,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174022
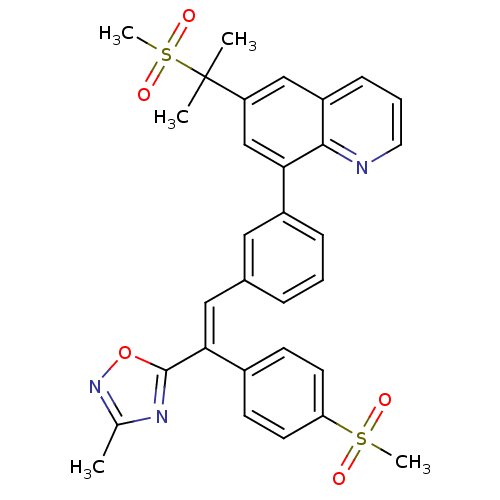
((E)-8-(3-(2-(3-methyl-1,2,4-oxadiazol-5-yl)-2-(4-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H29N3O5S2/c1-20-33-30(39-34-20)28(22-11-13-26(14-12-22)40(4,35)36)17-21-8-6-9-23(16-21)27-19-25(31(2,3)41(5,37)38)18-24-10-7-15-32-29(24)27/h6-19H,1-5H3/b28-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274032
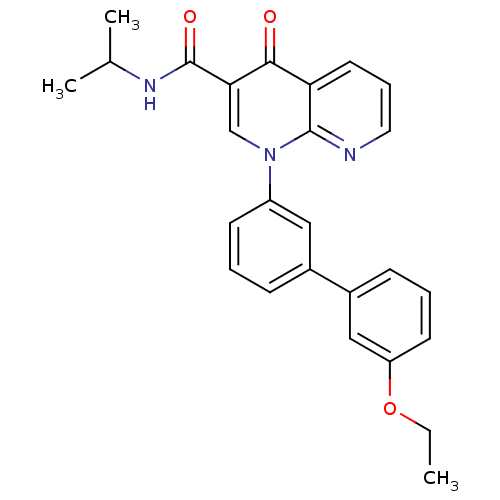
(1-(3'-Ethoxy-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CCOc1cccc(c1)-c1cccc(c1)-n1cc(C(=O)NC(C)C)c(=O)c2cccnc12 Show InChI InChI=1S/C26H25N3O3/c1-4-32-21-11-6-9-19(15-21)18-8-5-10-20(14-18)29-16-23(26(31)28-17(2)3)24(30)22-12-7-13-27-25(22)29/h5-17H,4H2,1-3H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274785
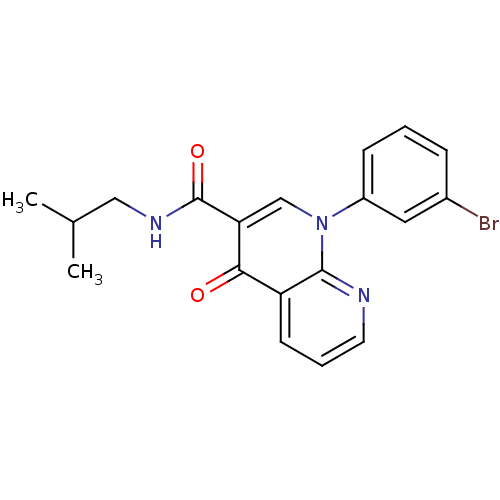
(1-(3-bromophenyl)-N-isobutyl-4-oxo-1,4-dihydro-1,8...)Show SMILES CC(C)CNC(=O)c1cn(-c2cccc(Br)c2)c2ncccc2c1=O Show InChI InChI=1S/C19H18BrN3O2/c1-12(2)10-22-19(25)16-11-23(14-6-3-5-13(20)9-14)18-15(17(16)24)7-4-8-21-18/h3-9,11-12H,10H2,1-2H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274228

(1-{3-[6-(1-Hydroxy-1-methyl-ethyl)-1-oxy-pyridin-3...)Show SMILES CC(C)(O)c1ccc(c[n+]1[O-])-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C26H24N4O4/c1-26(2,33)22-11-8-17(14-30(22)34)16-5-3-6-19(13-16)29-15-21(25(32)28-18-9-10-18)23(31)20-7-4-12-27-24(20)29/h3-8,11-15,18,33H,9-10H2,1-2H3,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274267
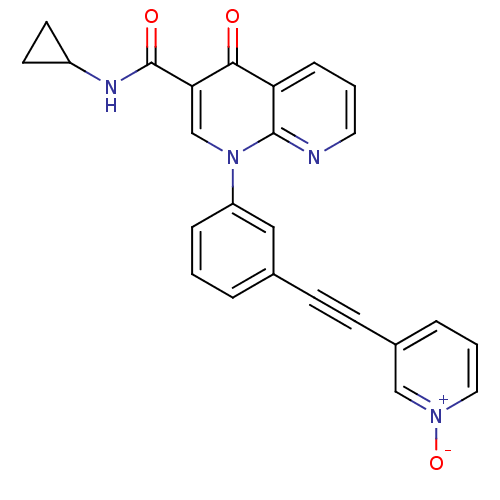
(4-Oxo-1-[3-(1-oxy-pyridin-3-ylethynyl)-phenyl]-1,4...)Show SMILES [O-][n+]1cccc(c1)C#Cc1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C25H18N4O3/c30-23-21-7-2-12-26-24(21)29(16-22(23)25(31)27-19-10-11-19)20-6-1-4-17(14-20)8-9-18-5-3-13-28(32)15-18/h1-7,12-16,19H,10-11H2,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274029
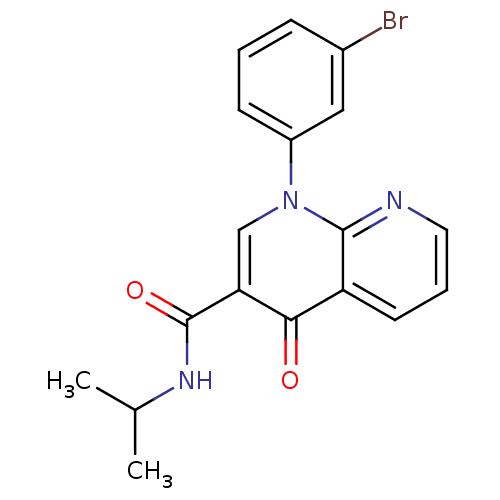
(1-(3-bromophenyl)-N-isopropyl-4-oxo-1,4-dihydro-1,...)Show InChI InChI=1S/C18H16BrN3O2/c1-11(2)21-18(24)15-10-22(13-6-3-5-12(19)9-13)17-14(16(15)23)7-4-8-20-17/h3-11H,1-2H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274030

(CHEMBL456173 | N-isopropyl-4-oxo-1-phenyl-1,4-dihy...)Show InChI InChI=1S/C18H17N3O2/c1-12(2)20-18(23)15-11-21(13-7-4-3-5-8-13)17-14(16(15)22)9-6-10-19-17/h3-12H,1-2H3,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274786
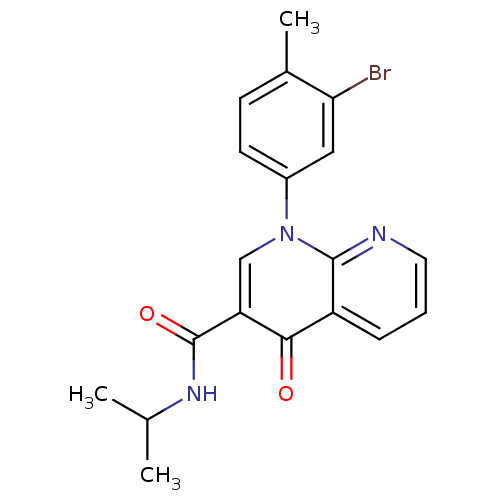
(1-(3-bromo-4-methylphenyl)-N-isopropyl-4-oxo-1,4-d...)Show SMILES CC(C)NC(=O)c1cn(-c2ccc(C)c(Br)c2)c2ncccc2c1=O Show InChI InChI=1S/C19H18BrN3O2/c1-11(2)22-19(25)15-10-23(13-7-6-12(3)16(20)9-13)18-14(17(15)24)5-4-8-21-18/h4-11H,1-3H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274788
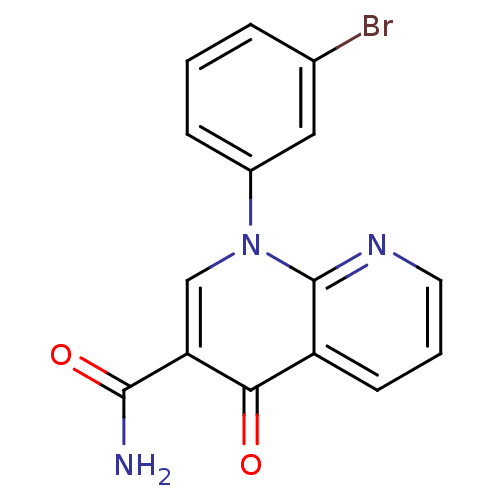
(1-(3-bromophenyl)-4-oxo-1,4-dihydro-1,8-naphthyrid...)Show InChI InChI=1S/C15H10BrN3O2/c16-9-3-1-4-10(7-9)19-8-12(14(17)21)13(20)11-5-2-6-18-15(11)19/h1-8H,(H2,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274027

(1-(4-chlorophenyl)-N-(3,5-dichloropyridin-4-yl)-4-...)Show SMILES Clc1ccc(cc1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C20H11Cl3N4O2/c21-11-3-5-12(6-4-11)27-10-14(18(28)13-2-1-7-25-19(13)27)20(29)26-17-15(22)8-24-9-16(17)23/h1-10H,(H,24,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274028

(CHEMBL499477 | N-(3,5-dichloropyridin-4-yl)-1-(3-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H22Cl2N6O5S/c1-18-37-32(45-39-18)24(20-8-10-22(11-9-20)46(2,43)44)14-19-5-3-6-21(13-19)40-17-25(29(41)23-7-4-12-36-30(23)40)31(42)38-28-26(33)15-35-16-27(28)34/h3-17H,1-2H3,(H,35,38,42)/b24-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274031

(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(=O)c1cccc(c1)-c1cccc(c1)-n1cc(C(=O)Nc2nccc(Cl)c2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C28H18Cl2N4O3/c1-16(35)17-5-2-6-18(13-17)19-7-3-8-20(14-19)34-15-22(25(36)21-9-4-11-32-27(21)34)28(37)33-26-24(30)23(29)10-12-31-26/h2-15H,1H3,(H,31,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274063

(1-(4'-Methylsulfanyl-biphenyl-3-yl)-4-oxo-1,4-dihy...)Show SMILES CSc1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NC(C)C)c(=O)c2cccnc12 Show InChI InChI=1S/C25H23N3O2S/c1-16(2)27-25(30)22-15-28(24-21(23(22)29)8-5-13-26-24)19-7-4-6-18(14-19)17-9-11-20(31-3)12-10-17/h4-16H,1-3H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274728
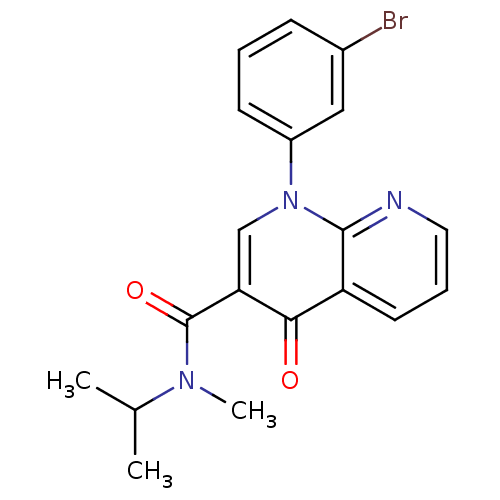
(1-(3-bromophenyl)-N-isopropyl-N-methyl-4-oxo-1,4-d...)Show SMILES CC(C)N(C)C(=O)c1cn(-c2cccc(Br)c2)c2ncccc2c1=O Show InChI InChI=1S/C19H18BrN3O2/c1-12(2)22(3)19(25)16-11-23(14-7-4-6-13(20)10-14)18-15(17(16)24)8-5-9-21-18/h4-12H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274787
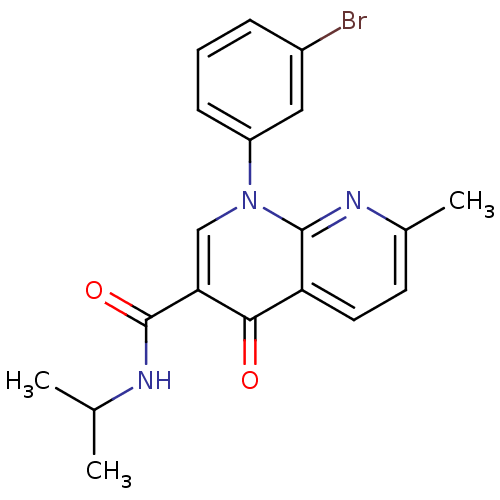
(1-(3-bromophenyl)-N-isopropyl-7-methyl-4-oxo-1,4-d...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(Br)c2)c2nc(C)ccc2c1=O Show InChI InChI=1S/C19H18BrN3O2/c1-11(2)21-19(25)16-10-23(14-6-4-5-13(20)9-14)18-15(17(16)24)8-7-12(3)22-18/h4-11H,1-3H3,(H,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274032

(1-(3'-Ethoxy-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CCOc1cccc(c1)-c1cccc(c1)-n1cc(C(=O)NC(C)C)c(=O)c2cccnc12 Show InChI InChI=1S/C26H25N3O3/c1-4-32-21-11-6-9-19(15-21)18-8-5-10-20(14-18)29-16-23(26(31)28-17(2)3)24(30)22-12-7-13-27-25(22)29/h5-17H,4H2,1-3H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274789

(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2cccc(c2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)11-6-12-27-25)21-10-5-9-20(14-21)19-8-4-7-18(13-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274188

(1-(4'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2ccc(cc2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)8-5-13-27-25)21-7-4-6-20(14-21)19-11-9-18(10-12-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274226
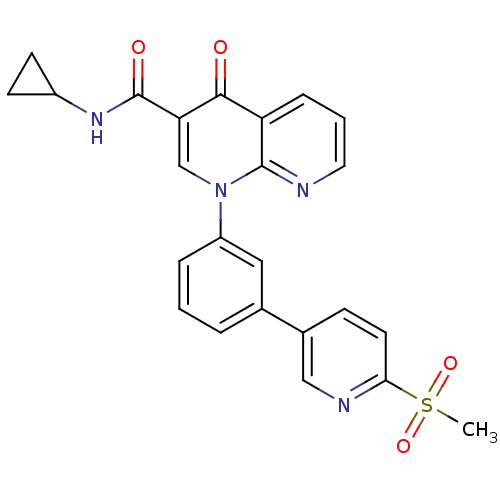
(CHEMBL484931 | N-cyclopropyl-1-(3-(6-(methylsulfon...)Show SMILES CS(=O)(=O)c1ccc(cn1)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C24H20N4O4S/c1-33(31,32)21-10-7-16(13-26-21)15-4-2-5-18(12-15)28-14-20(24(30)27-17-8-9-17)22(29)19-6-3-11-25-23(19)28/h2-7,10-14,17H,8-9H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274229
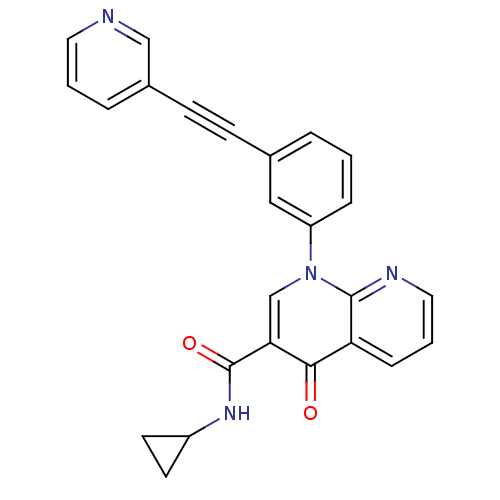
(CHEMBL521509 | N-cyclopropyl-4-oxo-1-(3-(2-(pyridi...)Show SMILES O=C(NC1CC1)c1cn(-c2cccc(c2)C#Cc2cccnc2)c2ncccc2c1=O Show InChI InChI=1S/C25H18N4O2/c30-23-21-7-3-13-27-24(21)29(16-22(23)25(31)28-19-10-11-19)20-6-1-4-17(14-20)8-9-18-5-2-12-26-15-18/h1-7,12-16,19H,10-11H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274190
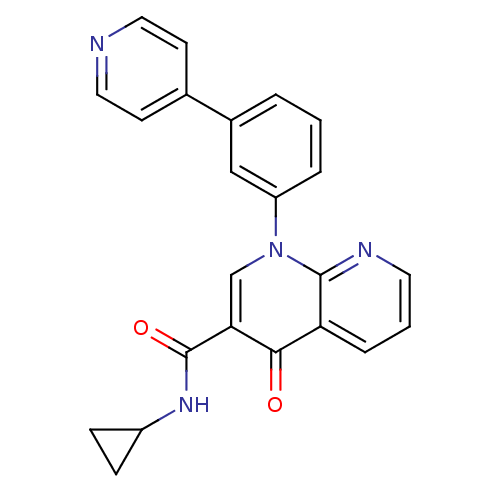
(CHEMBL484754 | N-cyclopropyl-4-oxo-1-(3-(pyridin-4...)Show SMILES O=C(NC1CC1)c1cn(-c2cccc(c2)-c2ccncc2)c2ncccc2c1=O Show InChI InChI=1S/C23H18N4O2/c28-21-19-5-2-10-25-22(19)27(14-20(21)23(29)26-17-6-7-17)18-4-1-3-16(13-18)15-8-11-24-12-9-15/h1-5,8-14,17H,6-7H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274189

(1-(4'-Methanesulfonyl-biphenyl-3-yl)-4-oxo-1,4-dih...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2ccc(cc2)S(C)(=O)=O)c2ncccc2c1=O Show InChI InChI=1S/C25H23N3O4S/c1-16(2)27-25(30)22-15-28(24-21(23(22)29)8-5-13-26-24)19-7-4-6-18(14-19)17-9-11-20(12-10-17)33(3,31)32/h4-16H,1-3H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274029

(1-(3-bromophenyl)-N-isopropyl-4-oxo-1,4-dihydro-1,...)Show InChI InChI=1S/C18H16BrN3O2/c1-11(2)21-18(24)15-10-22(13-6-3-5-12(19)9-13)17-14(16(15)23)7-4-8-20-17/h3-11H,1-2H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274785

(1-(3-bromophenyl)-N-isobutyl-4-oxo-1,4-dihydro-1,8...)Show SMILES CC(C)CNC(=O)c1cn(-c2cccc(Br)c2)c2ncccc2c1=O Show InChI InChI=1S/C19H18BrN3O2/c1-12(2)10-22-19(25)16-11-23(14-6-3-5-13(20)9-14)18-15(17(16)24)7-4-8-21-18/h3-9,11-12H,10H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274191
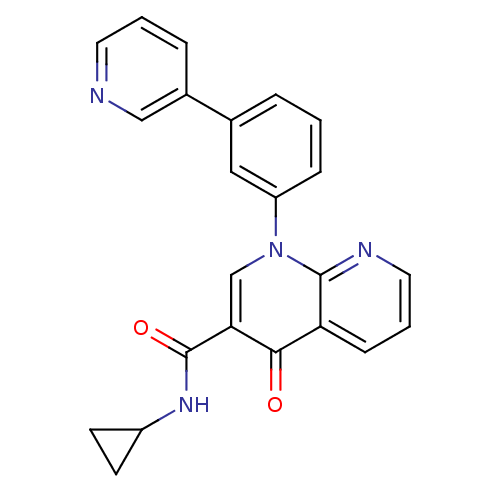
(CHEMBL519355 | N-cyclopropyl-4-oxo-1-(3-(pyridin-3...)Show SMILES O=C(NC1CC1)c1cn(-c2cccc(c2)-c2cccnc2)c2ncccc2c1=O Show InChI InChI=1S/C23H18N4O2/c28-21-19-7-3-11-25-22(19)27(14-20(21)23(29)26-17-8-9-17)18-6-1-4-15(12-18)16-5-2-10-24-13-16/h1-7,10-14,17H,8-9H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274227
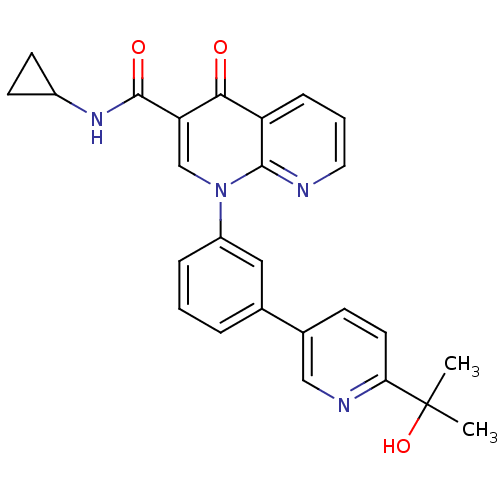
(CHEMBL520502 | N-cyclopropyl-1-(3-(6-(2-hydroxypro...)Show SMILES CC(C)(O)c1ccc(cn1)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C26H24N4O3/c1-26(2,33)22-11-8-17(14-28-22)16-5-3-6-19(13-16)30-15-21(25(32)29-18-9-10-18)23(31)20-7-4-12-27-24(20)30/h3-8,11-15,18,33H,9-10H2,1-2H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50274267

(4-Oxo-1-[3-(1-oxy-pyridin-3-ylethynyl)-phenyl]-1,4...)Show SMILES [O-][n+]1cccc(c1)C#Cc1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C25H18N4O3/c30-23-21-7-2-12-26-24(21)29(16-22(23)25(31)27-19-10-11-19)20-6-1-4-17(14-20)8-9-18-5-3-13-28(32)15-18/h1-7,12-16,19H,10-11H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50174022

((E)-8-(3-(2-(3-methyl-1,2,4-oxadiazol-5-yl)-2-(4-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H29N3O5S2/c1-20-33-30(39-34-20)28(22-11-13-26(14-12-22)40(4,35)36)17-21-8-6-9-23(16-21)27-19-25(31(2,3)41(5,37)38)18-24-10-7-15-32-29(24)27/h6-19H,1-5H3/b28-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274228

(1-{3-[6-(1-Hydroxy-1-methyl-ethyl)-1-oxy-pyridin-3...)Show SMILES CC(C)(O)c1ccc(c[n+]1[O-])-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C26H24N4O4/c1-26(2,33)22-11-8-17(14-30(22)34)16-5-3-6-19(13-16)29-15-21(25(32)28-18-9-10-18)23(31)20-7-4-12-27-24(20)29/h3-8,11-15,18,33H,9-10H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50274228

(1-{3-[6-(1-Hydroxy-1-methyl-ethyl)-1-oxy-pyridin-3...)Show SMILES CC(C)(O)c1ccc(c[n+]1[O-])-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C26H24N4O4/c1-26(2,33)22-11-8-17(14-30(22)34)16-5-3-6-19(13-16)29-15-21(25(32)28-18-9-10-18)23(31)20-7-4-12-27-24(20)29/h3-8,11-15,18,33H,9-10H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274267

(4-Oxo-1-[3-(1-oxy-pyridin-3-ylethynyl)-phenyl]-1,4...)Show SMILES [O-][n+]1cccc(c1)C#Cc1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C25H18N4O3/c30-23-21-7-2-12-26-24(21)29(16-22(23)25(31)27-19-10-11-19)20-6-1-4-17(14-20)8-9-18-5-3-13-28(32)15-18/h1-7,12-16,19H,10-11H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274030

(CHEMBL456173 | N-isopropyl-4-oxo-1-phenyl-1,4-dihy...)Show InChI InChI=1S/C18H17N3O2/c1-12(2)20-18(23)15-11-21(13-7-4-3-5-8-13)17-14(16(15)22)9-6-10-19-17/h3-12H,1-2H3,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274268
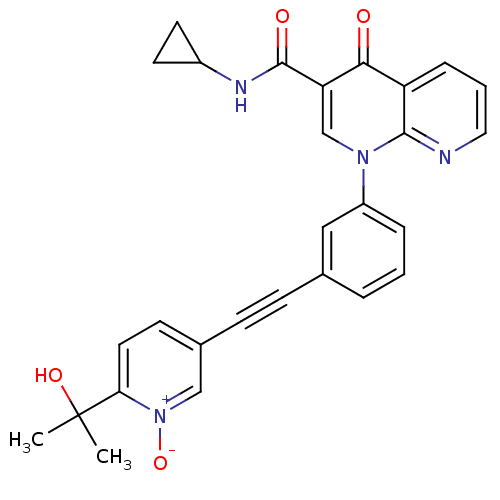
(1-{3-[6-(1-Hydroxy-1-methyl-ethyl)-1-oxy-pyridin-3...)Show SMILES CC(C)(O)c1ccc(c[n+]1[O-])C#Cc1cccc(c1)-[n+]1cc(C(=O)NC2CC2)c([O-])c2cccnc12 Show InChI InChI=1S/C28H24N4O4/c1-28(2,35)24-13-10-19(16-32(24)36)9-8-18-5-3-6-21(15-18)31-17-23(27(34)30-20-11-12-20)25(33)22-7-4-14-29-26(22)31/h3-7,10,13-17,20,35H,11-12H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50274788

(1-(3-bromophenyl)-4-oxo-1,4-dihydro-1,8-naphthyrid...)Show InChI InChI=1S/C15H10BrN3O2/c16-9-3-1-4-10(7-9)19-8-12(14(17)21)13(20)11-5-2-6-18-15(11)19/h1-8H,(H2,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >9.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data