

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274200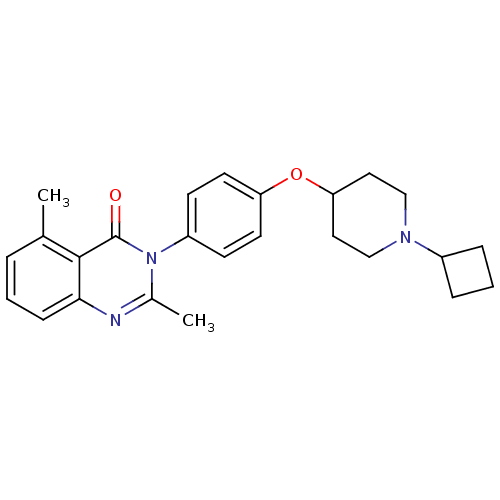 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274235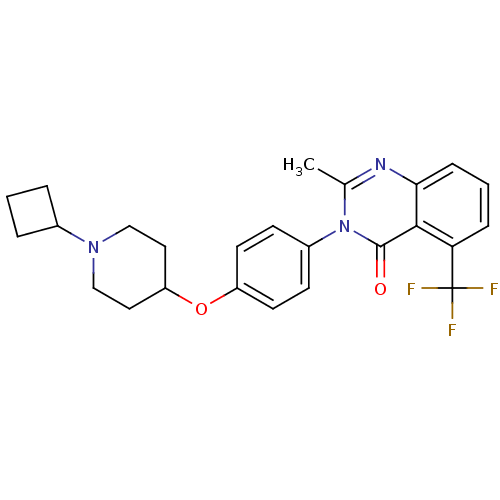 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in HEK293 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO-K1 cells | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274200 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274798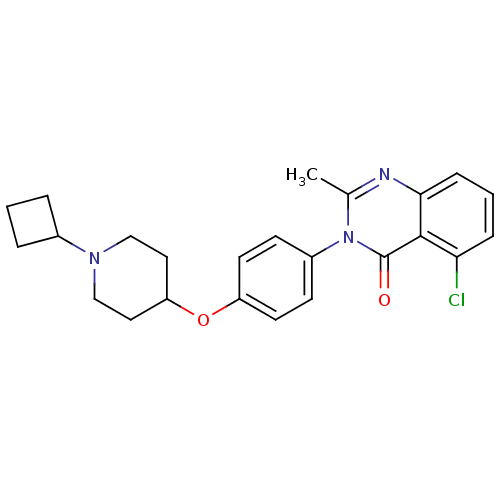 (5-Chloro-3-(4-[(1-cyclobutyl-4-piperidinyl)oxy]phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247054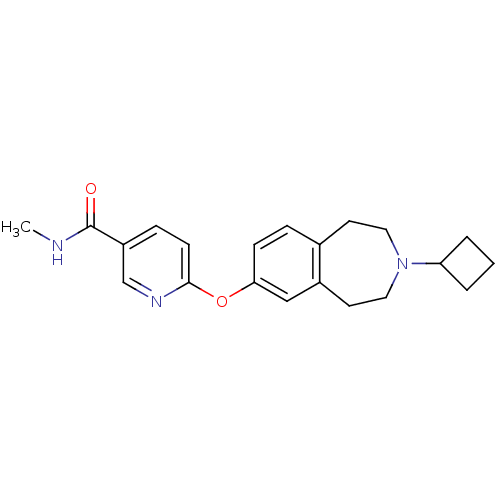 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274736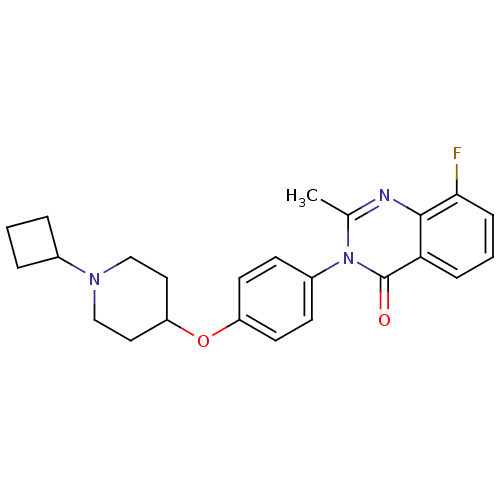 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-8-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274737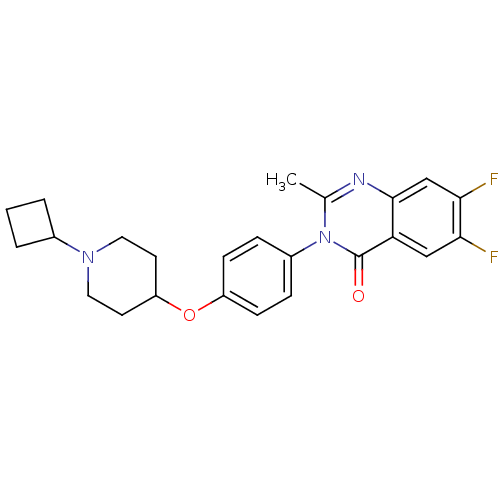 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-6,7-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274037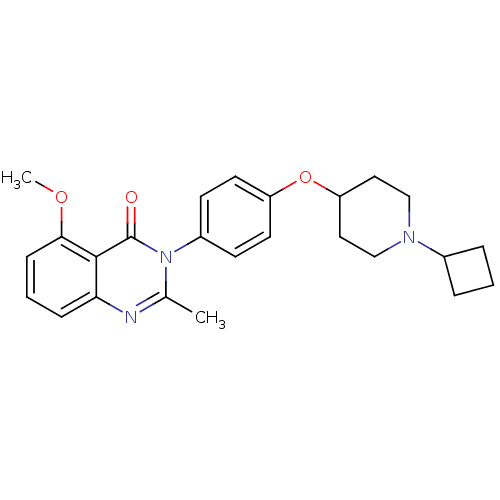 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274734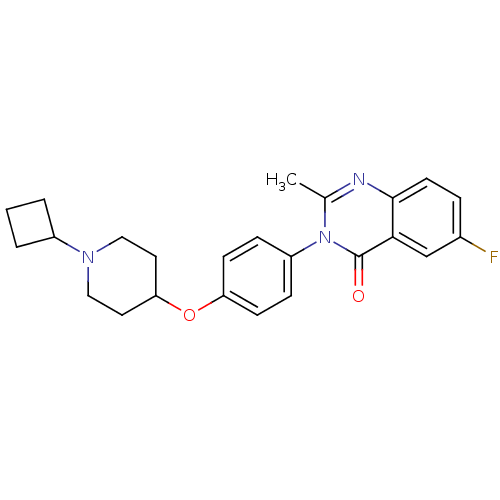 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-6-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274735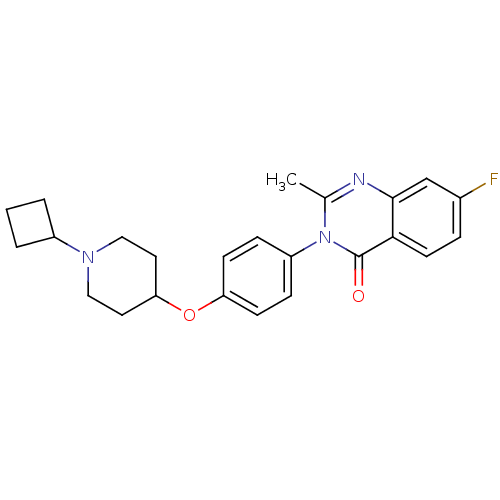 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-7-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274616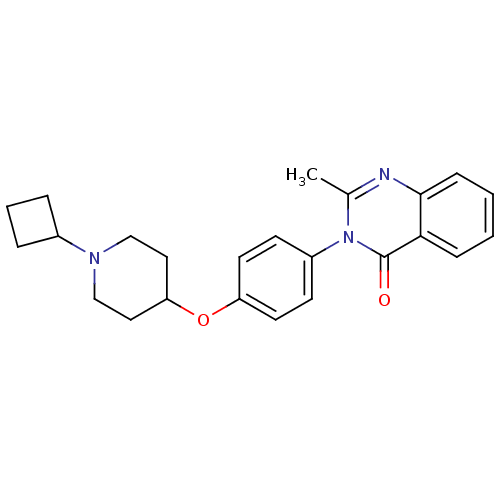 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274799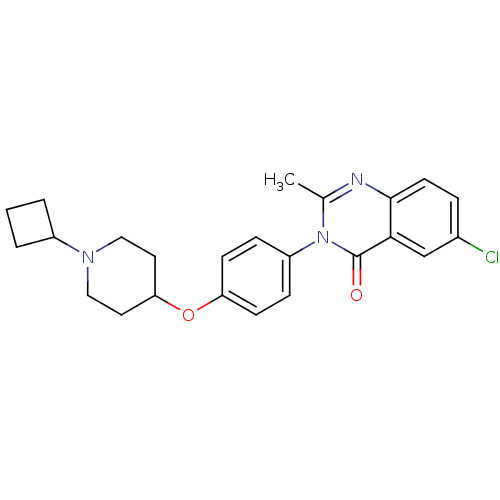 (6-Chloro-3-(4-[(1-cyclobutyl-4-piperidinyl)oxy]phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274038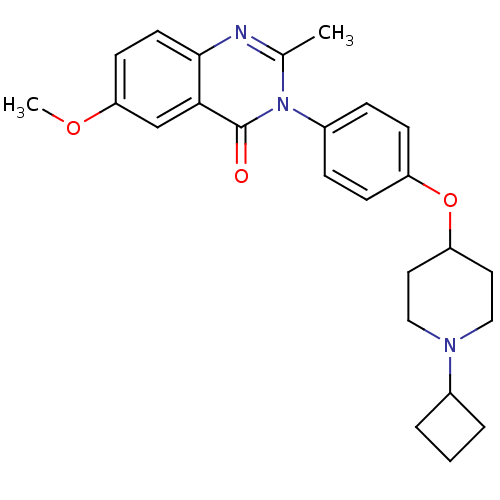 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-6-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274199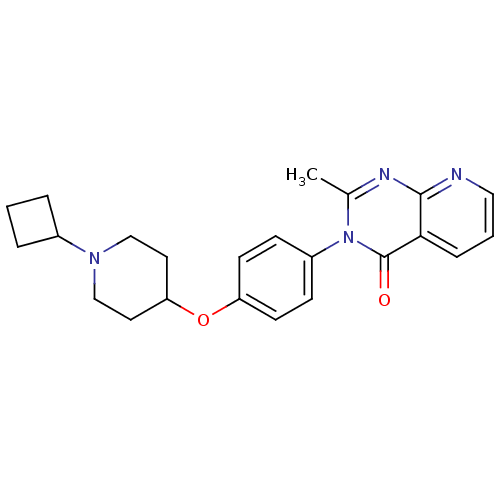 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274801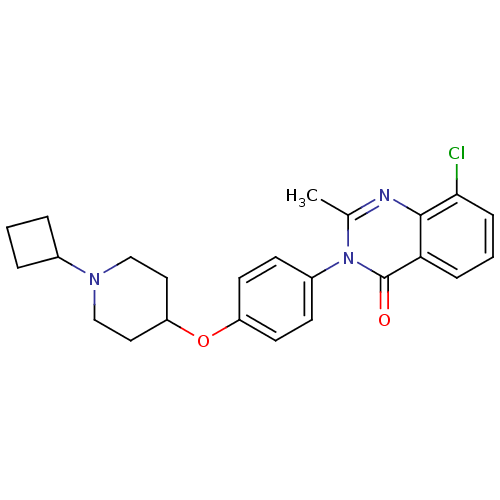 (8-Chloro-3-(4-[(1-cyclobutyl-4-piperidinyl)oxy]phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50263293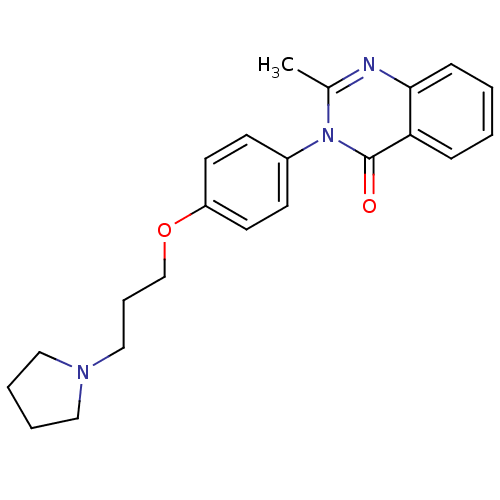 (2-Methyl-3-(4-{[3-(1-pyrrolidinyl)propyl]oxy}pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274039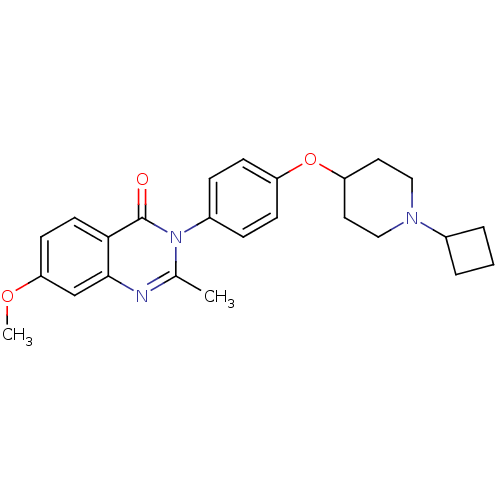 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-7-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274198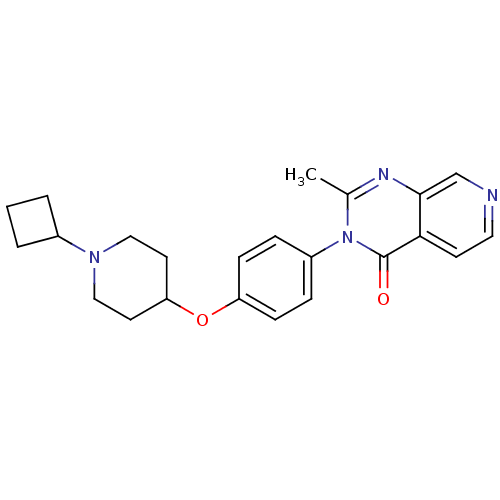 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274197 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274800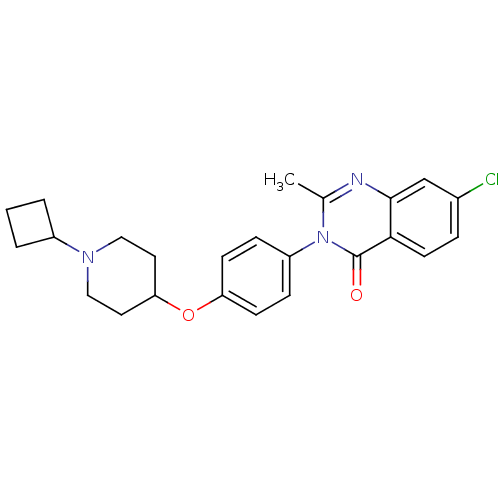 (7-Chloro-3-(4-[(1-cyclobutyl-4-piperidinyl)oxy]phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274617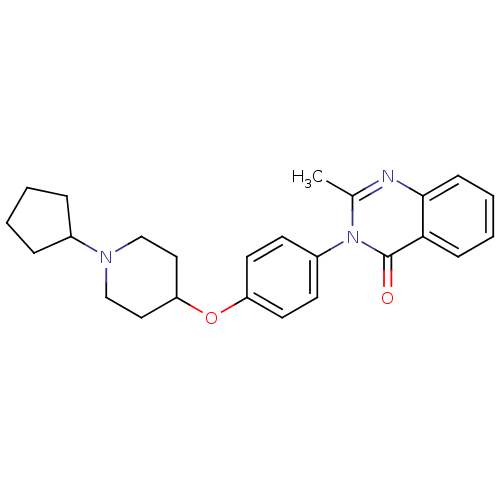 (3-(4-[(1-Cyclopentyl-4-piperidinyl)oxy]phenyl)-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274654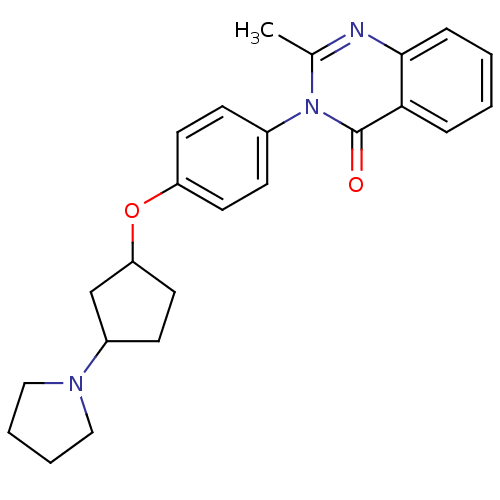 (2-Methyl-3-[4-([3-(1-pyrrolidinyl)cyclopentyl]oxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274654 (2-Methyl-3-[4-([3-(1-pyrrolidinyl)cyclopentyl]oxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274040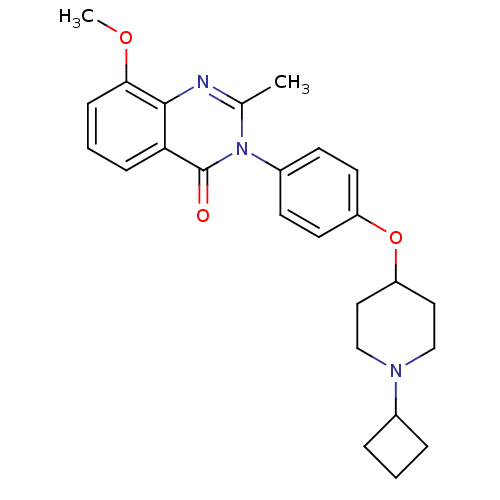 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-8-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274653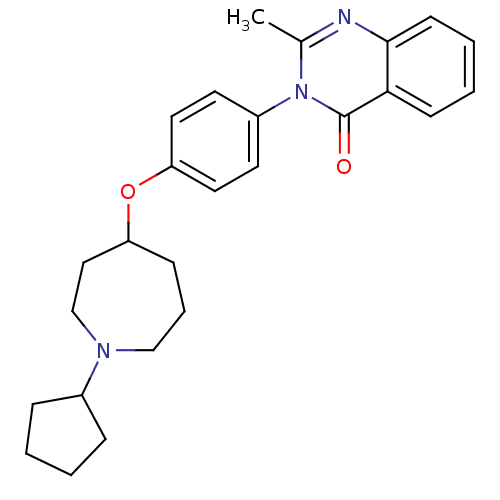 (3-(4-[(1-Cyclopentyl-4-azepanyl)oxy]phenyl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274690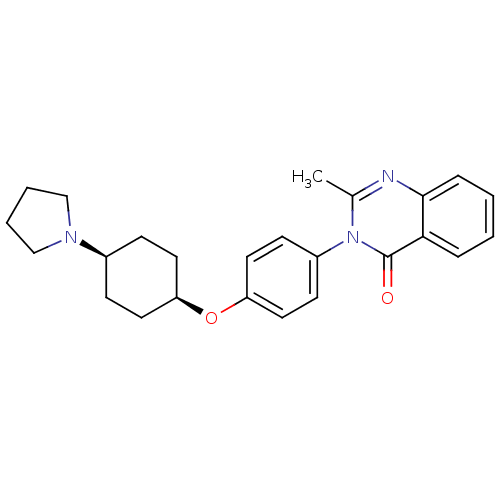 (CHEMBL521022 | cis-2-Methyl-3-(4-([4-(1-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274652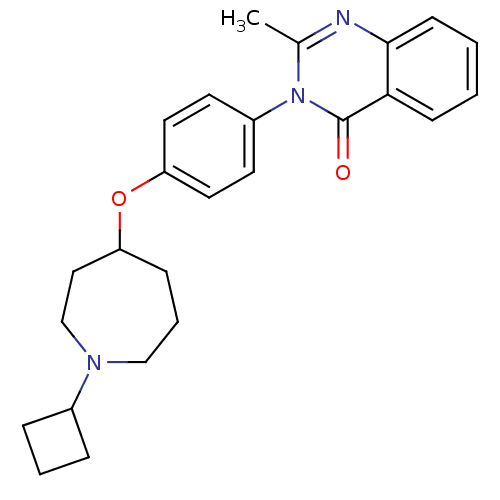 (3-(4-[(1-Cyclobutyl-4-azepanyl)oxy]phenyl)-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274077 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274579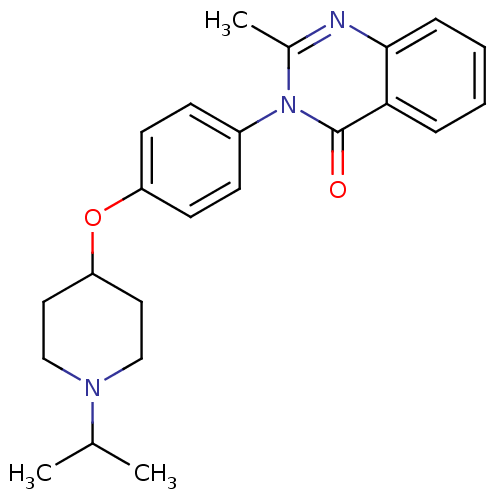 (3-(4-[(1-Isopropyl-4-piperidinyl)oxy]phenyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274651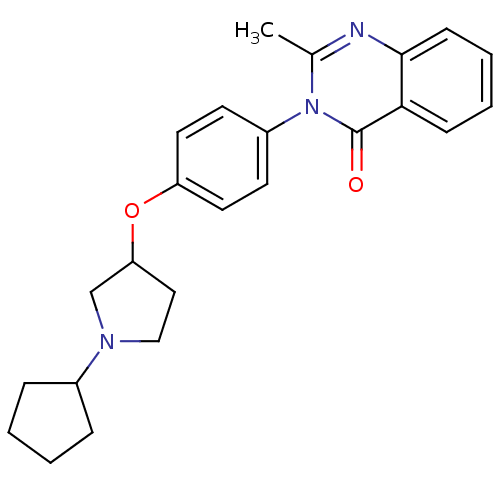 (3-(4-[(1-Cyclopentyl-3-pyrrolidinyl)oxy]phenyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274618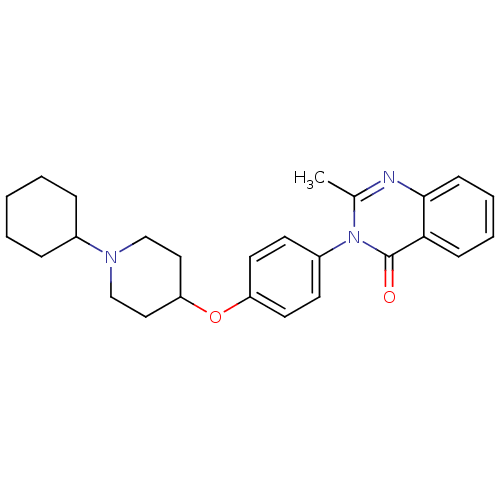 (3-(4-[(1-Cyclohexyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274576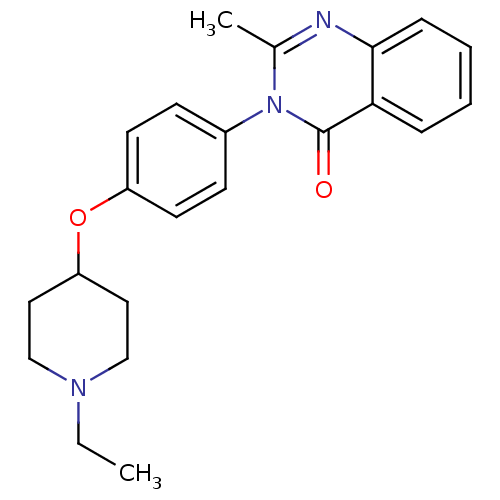 (3-(4-[(1-Ethyl-4-piperidinyl)oxy]phenyl)-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274577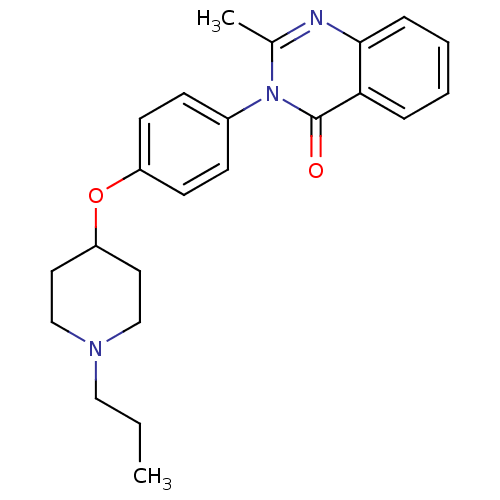 (2-Methyl-3-(4-[(1-propyl-4-piperidinyl)oxy]phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274578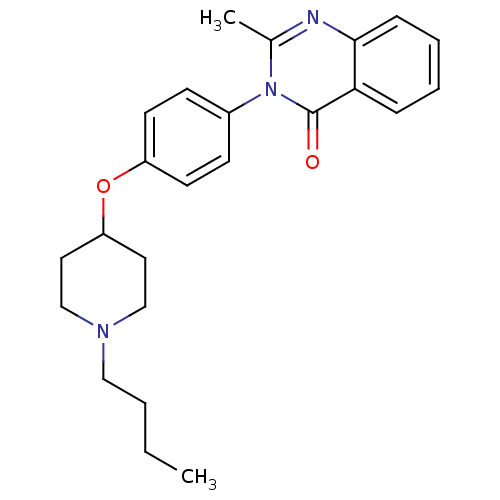 (3-(4-[(1-Butyl-4-piperidinyl)oxy]phenyl)-2-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274619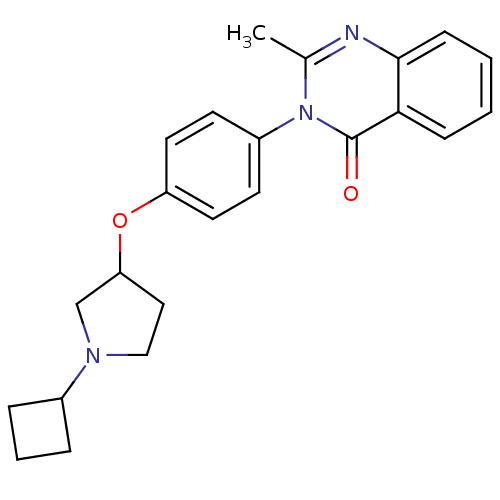 (3-(4-[(1-Cyclobutyl-3-pyrrolidinyl)oxy]phenyl)-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274575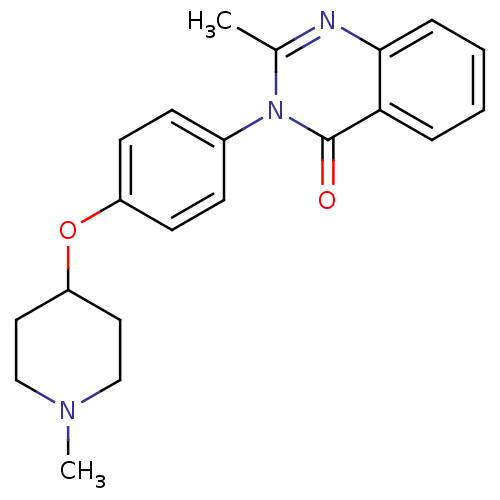 (2-Methyl-3-(4-[(1-methyl-4-piperidinyl)oxy]phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274691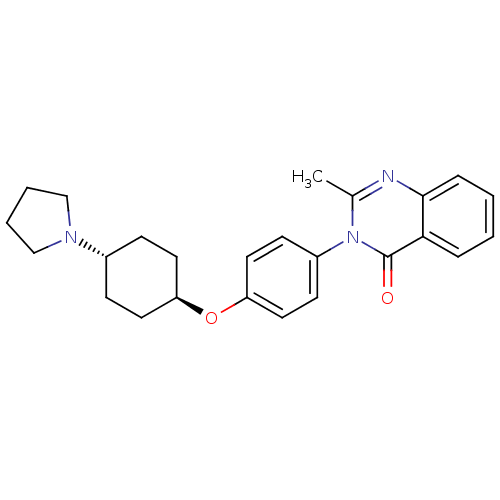 (CHEMBL485604 | trans-2-Methyl-3-(4-([4-(1-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 813 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50274539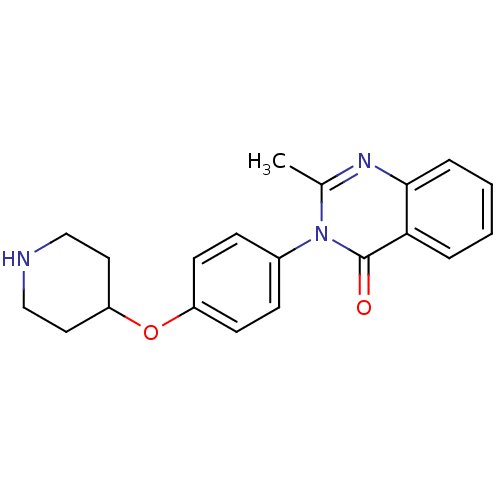 (2-Methyl-3-[4-(4-piperidinyloxy)phenyl]-4(3H)-quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned histamine H3 receptor expressed in CHO-K1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]GT... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50274801 (8-Chloro-3-(4-[(1-cyclobutyl-4-piperidinyl)oxy]phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ERG in HEK293 cells assessed as inhibition of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihyd... | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |