 Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50027495
Found 60 hits Enz. Inhib. hit(s) with all data for entry = 50027495 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246381
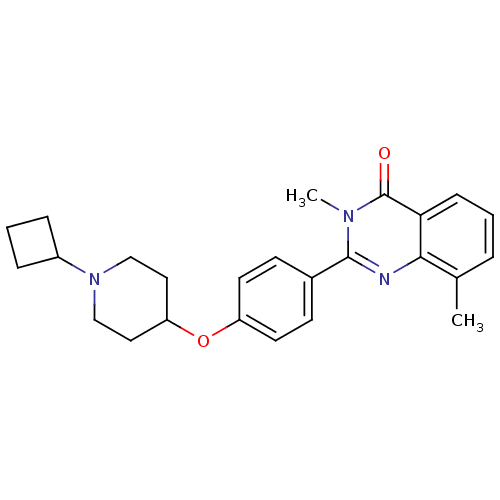
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3,8-di...)Show SMILES Cc1cccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C25H29N3O2/c1-17-5-3-8-22-23(17)26-24(27(2)25(22)29)18-9-11-20(12-10-18)30-21-13-15-28(16-14-21)19-6-4-7-19/h3,5,8-12,19,21H,4,6-7,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246382
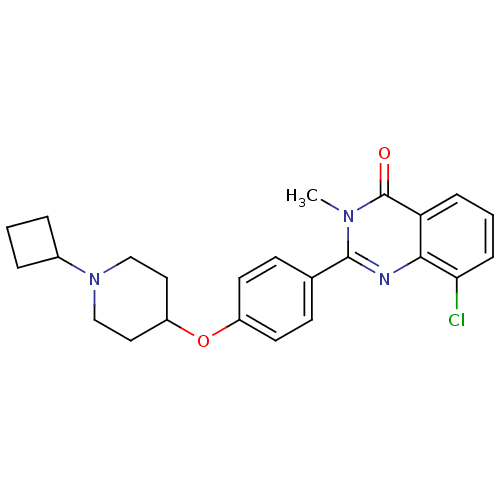
(8-chloro-2-(4-(1-cyclobutylpiperidin-4-yloxy)pheny...)Show SMILES Cn1c(nc2c(Cl)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26ClN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246290
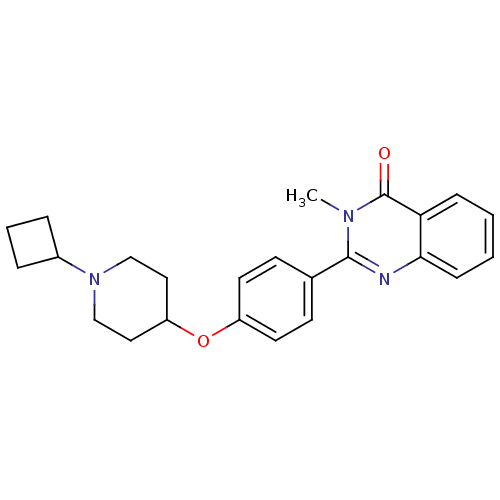
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H27N3O2/c1-26-23(25-22-8-3-2-7-21(22)24(26)28)17-9-11-19(12-10-17)29-20-13-15-27(16-14-20)18-5-4-6-18/h2-3,7-12,18,20H,4-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246434
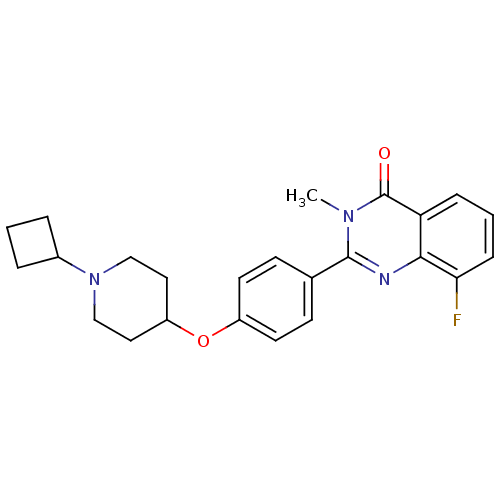
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-fluo...)Show SMILES Cn1c(nc2c(F)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26FN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246333
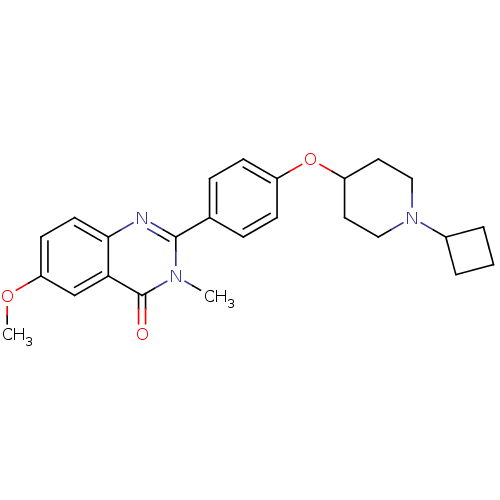
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-6-meth...)Show SMILES COc1ccc2nc(-c3ccc(OC4CCN(CC4)C4CCC4)cc3)n(C)c(=O)c2c1 Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-11-10-21(30-2)16-22(23)25(27)29)17-6-8-19(9-7-17)31-20-12-14-28(15-13-20)18-4-3-5-18/h6-11,16,18,20H,3-5,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246435
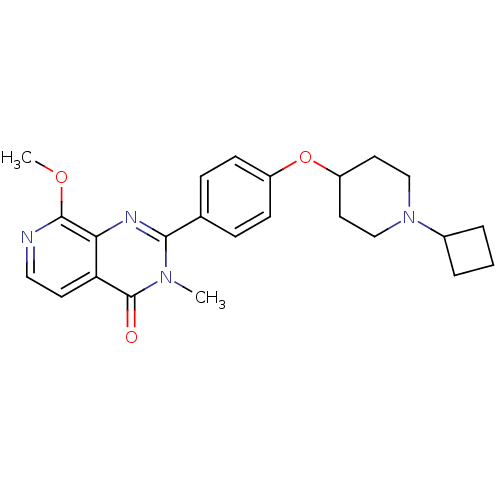
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...)Show SMILES COc1nccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C24H28N4O3/c1-27-22(26-21-20(24(27)29)10-13-25-23(21)30-2)16-6-8-18(9-7-16)31-19-11-14-28(15-12-19)17-4-3-5-17/h6-10,13,17,19H,3-5,11-12,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246287
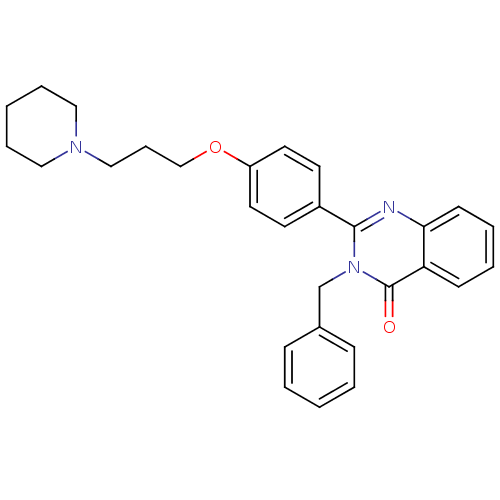
(3-benzyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(Cc2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H31N3O2/c33-29-26-12-5-6-13-27(26)30-28(32(29)22-23-10-3-1-4-11-23)24-14-16-25(17-15-24)34-21-9-20-31-18-7-2-8-19-31/h1,3-6,10-17H,2,7-9,18-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246334
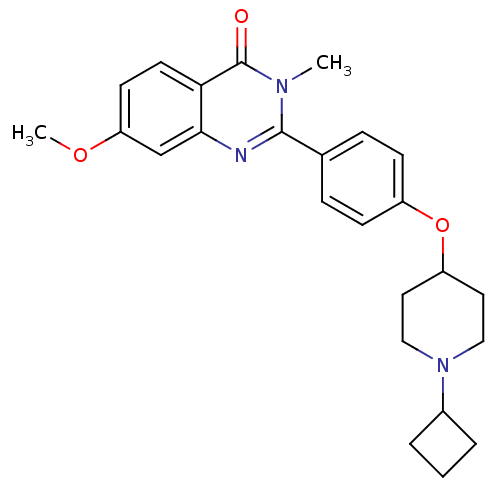
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-7-meth...)Show SMILES COc1ccc2c(c1)nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-16-21(30-2)10-11-22(23)25(27)29)17-6-8-19(9-7-17)31-20-12-14-28(15-13-20)18-4-3-5-18/h6-11,16,18,20H,3-5,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246243
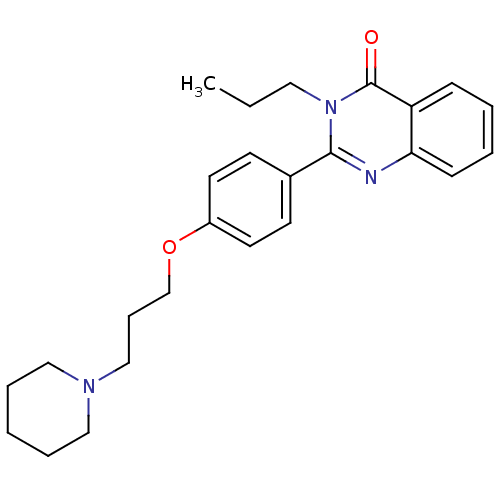
(2-(4-(3-(piperidin-1-yl)propoxy)phenyl)-3-propylqu...)Show SMILES CCCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-2-15-28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-19-8-18-27-16-6-3-7-17-27/h4-5,9-14H,2-3,6-8,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246380
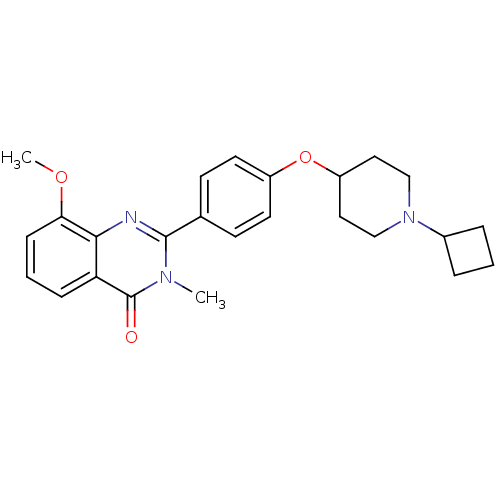
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...)Show SMILES COc1cccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-21(25(27)29)7-4-8-22(23)30-2)17-9-11-19(12-10-17)31-20-13-15-28(16-14-20)18-5-3-6-18/h4,7-12,18,20H,3,5-6,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246332
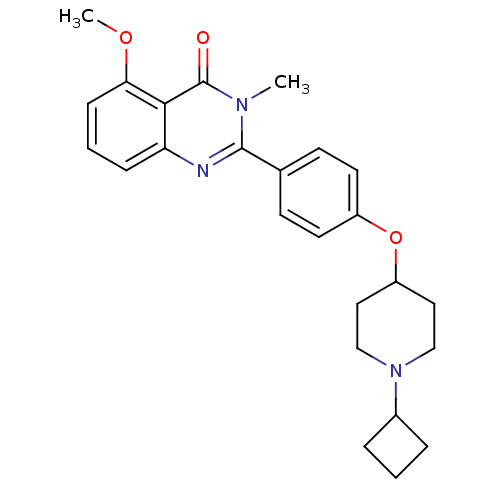
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-5-meth...)Show SMILES COc1cccc2nc(-c3ccc(OC4CCN(CC4)C4CCC4)cc3)n(C)c(=O)c12 Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-21-7-4-8-22(30-2)23(21)25(27)29)17-9-11-19(12-10-17)31-20-13-15-28(16-14-20)18-5-3-6-18/h4,7-12,18,20H,3,5-6,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246289
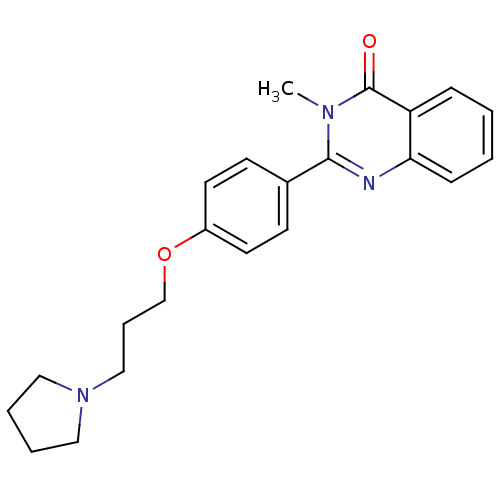
(3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...)Show InChI InChI=1S/C22H25N3O2/c1-24-21(23-20-8-3-2-7-19(20)22(24)26)17-9-11-18(12-10-17)27-16-6-15-25-13-4-5-14-25/h2-3,7-12H,4-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246383
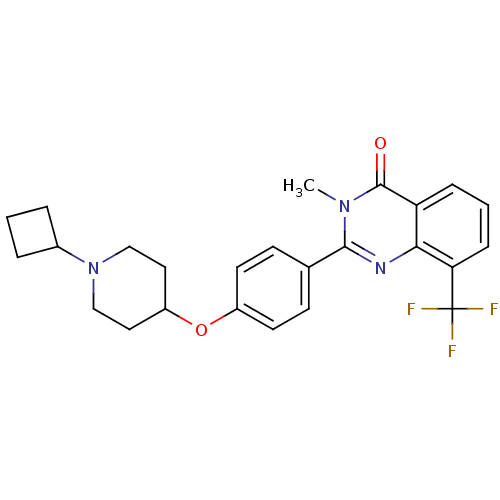
(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...)Show SMILES Cn1c(nc2c(cccc2c1=O)C(F)(F)F)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C25H26F3N3O2/c1-30-23(29-22-20(24(30)32)6-3-7-21(22)25(26,27)28)16-8-10-18(11-9-16)33-19-12-14-31(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246331
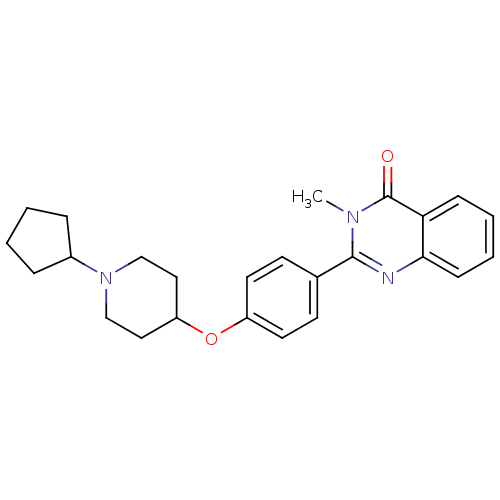
(2-(4-(1-cyclopentylpiperidin-4-yloxy)phenyl)-3-met...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C25H29N3O2/c1-27-24(26-23-9-5-4-8-22(23)25(27)29)18-10-12-20(13-11-18)30-21-14-16-28(17-15-21)19-6-2-3-7-19/h4-5,8-13,19,21H,2-3,6-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246242
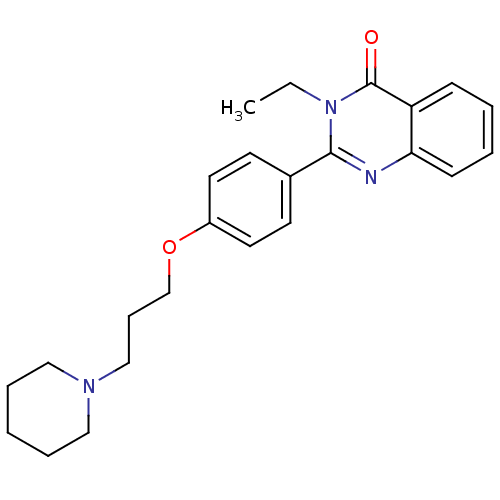
(3-ethyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qui...)Show SMILES CCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C24H29N3O2/c1-2-27-23(25-22-10-5-4-9-21(22)24(27)28)19-11-13-20(14-12-19)29-18-8-17-26-15-6-3-7-16-26/h4-5,9-14H,2-3,6-8,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246244

(3-isopropyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl...)Show SMILES CC(C)n1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-19(2)28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-18-8-17-27-15-6-3-7-16-27/h4-5,9-14,19H,3,6-8,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246241
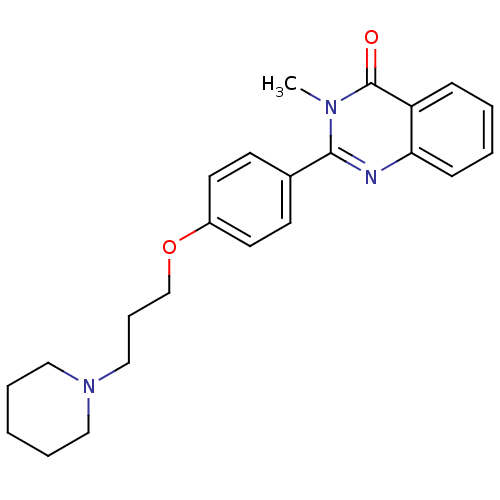
(3-methyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show InChI InChI=1S/C23H27N3O2/c1-25-22(24-21-9-4-3-8-20(21)23(25)27)18-10-12-19(13-11-18)28-17-7-16-26-14-5-2-6-15-26/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246288
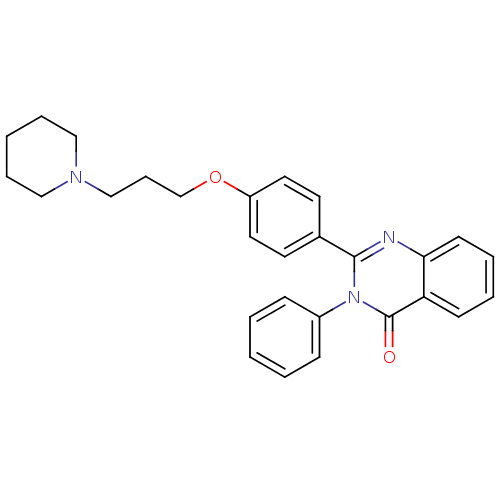
(3-phenyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(-c2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H29N3O2/c32-28-25-12-5-6-13-26(25)29-27(31(28)23-10-3-1-4-11-23)22-14-16-24(17-15-22)33-21-9-20-30-18-7-2-8-19-30/h1,3-6,10-17H,2,7-9,18-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50246240
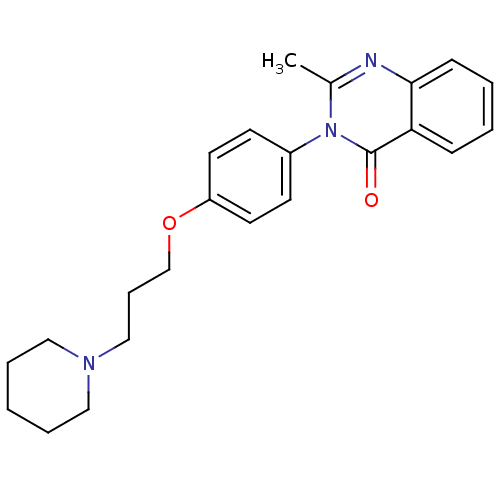
(2-Methyl-3-(4-{[3-(1-piperidinyl)propyl]oxy}phenyl...)Show InChI InChI=1S/C23H27N3O2/c1-18-24-22-9-4-3-8-21(22)23(27)26(18)19-10-12-20(13-11-19)28-17-7-16-25-14-5-2-6-15-25/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human histamine H3 receptor assessed as inhibition of R-alpha-methylhistamine-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246287

(3-benzyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(Cc2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H31N3O2/c33-29-26-12-5-6-13-27(26)30-28(32(29)22-23-10-3-1-4-11-23)24-14-16-25(17-15-24)34-21-9-20-31-18-7-2-8-19-31/h1,3-6,10-17H,2,7-9,18-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246383

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...)Show SMILES Cn1c(nc2c(cccc2c1=O)C(F)(F)F)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C25H26F3N3O2/c1-30-23(29-22-20(24(30)32)6-3-7-21(22)25(26,27)28)16-8-10-18(11-9-16)33-19-12-14-31(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246288

(3-phenyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(-c2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H29N3O2/c32-28-25-12-5-6-13-26(25)29-27(31(28)23-10-3-1-4-11-23)22-14-16-24(17-15-22)33-21-9-20-30-18-7-2-8-19-30/h1,3-6,10-17H,2,7-9,18-21H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246244

(3-isopropyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl...)Show SMILES CC(C)n1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-19(2)28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-18-8-17-27-15-6-3-7-16-27/h4-5,9-14,19H,3,6-8,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246382

(8-chloro-2-(4-(1-cyclobutylpiperidin-4-yloxy)pheny...)Show SMILES Cn1c(nc2c(Cl)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26ClN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246242

(3-ethyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qui...)Show SMILES CCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C24H29N3O2/c1-2-27-23(25-22-10-5-4-9-21(22)24(27)28)19-11-13-20(14-12-19)29-18-8-17-26-15-6-3-7-16-26/h4-5,9-14H,2-3,6-8,15-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246242

(3-ethyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qui...)Show SMILES CCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C24H29N3O2/c1-2-27-23(25-22-10-5-4-9-21(22)24(27)28)19-11-13-20(14-12-19)29-18-8-17-26-15-6-3-7-16-26/h4-5,9-14H,2-3,6-8,15-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246243

(2-(4-(3-(piperidin-1-yl)propoxy)phenyl)-3-propylqu...)Show SMILES CCCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-2-15-28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-19-8-18-27-16-6-3-7-17-27/h4-5,9-14H,2-3,6-8,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246243

(2-(4-(3-(piperidin-1-yl)propoxy)phenyl)-3-propylqu...)Show SMILES CCCn1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-2-15-28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-19-8-18-27-16-6-3-7-17-27/h4-5,9-14H,2-3,6-8,15-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246381

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3,8-di...)Show SMILES Cc1cccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C25H29N3O2/c1-17-5-3-8-22-23(17)26-24(27(2)25(22)29)18-9-11-20(12-10-18)30-21-13-15-28(16-14-21)19-6-4-7-19/h3,5,8-12,19,21H,4,6-7,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246289

(3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...)Show InChI InChI=1S/C22H25N3O2/c1-24-21(23-20-8-3-2-7-19(20)22(24)26)17-9-11-18(12-10-17)27-16-6-15-25-13-4-5-14-25/h2-3,7-12H,4-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246288

(3-phenyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(-c2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H29N3O2/c32-28-25-12-5-6-13-26(25)29-27(31(28)23-10-3-1-4-11-23)22-14-16-24(17-15-22)33-21-9-20-30-18-7-2-8-19-30/h1,3-6,10-17H,2,7-9,18-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246241

(3-methyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show InChI InChI=1S/C23H27N3O2/c1-25-22(24-21-9-4-3-8-20(21)23(25)27)18-10-12-19(13-11-18)28-17-7-16-26-14-5-2-6-15-26/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246289

(3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...)Show InChI InChI=1S/C22H25N3O2/c1-24-21(23-20-8-3-2-7-19(20)22(24)26)17-9-11-18(12-10-17)27-16-6-15-25-13-4-5-14-25/h2-3,7-12H,4-6,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246331

(2-(4-(1-cyclopentylpiperidin-4-yloxy)phenyl)-3-met...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C25H29N3O2/c1-27-24(26-23-9-5-4-8-22(23)25(27)29)18-10-12-20(13-11-18)30-21-14-16-28(17-15-21)19-6-2-3-7-19/h4-5,8-13,19,21H,2-3,6-7,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246241

(3-methyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show InChI InChI=1S/C23H27N3O2/c1-25-22(24-21-9-4-3-8-20(21)23(25)27)18-10-12-19(13-11-18)28-17-7-16-26-14-5-2-6-15-26/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246244

(3-isopropyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl...)Show SMILES CC(C)n1c(nc2ccccc2c1=O)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C25H31N3O2/c1-19(2)28-24(26-23-10-5-4-9-22(23)25(28)29)20-11-13-21(14-12-20)30-18-8-17-27-15-6-3-7-16-27/h4-5,9-14,19H,3,6-8,15-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246333

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-6-meth...)Show SMILES COc1ccc2nc(-c3ccc(OC4CCN(CC4)C4CCC4)cc3)n(C)c(=O)c2c1 Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-11-10-21(30-2)16-22(23)25(27)29)17-6-8-19(9-7-17)31-20-12-14-28(15-13-20)18-4-3-5-18/h6-11,16,18,20H,3-5,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246332

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-5-meth...)Show SMILES COc1cccc2nc(-c3ccc(OC4CCN(CC4)C4CCC4)cc3)n(C)c(=O)c12 Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-21-7-4-8-22(30-2)23(21)25(27)29)17-9-11-19(12-10-17)31-20-13-15-28(16-14-20)18-5-3-6-18/h4,7-12,18,20H,3,5-6,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246334

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-7-meth...)Show SMILES COc1ccc2c(c1)nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-16-21(30-2)10-11-22(23)25(27)29)17-6-8-19(9-7-17)31-20-12-14-28(15-13-20)18-4-3-5-18/h6-11,16,18,20H,3-5,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246434

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-fluo...)Show SMILES Cn1c(nc2c(F)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26FN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246290

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H27N3O2/c1-26-23(25-22-8-3-2-7-21(22)24(26)28)17-9-11-19(12-10-17)29-20-13-15-27(16-14-20)18-5-4-6-18/h2-3,7-12,18,20H,4-6,13-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246333

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-6-meth...)Show SMILES COc1ccc2nc(-c3ccc(OC4CCN(CC4)C4CCC4)cc3)n(C)c(=O)c2c1 Show InChI InChI=1S/C25H29N3O3/c1-27-24(26-23-11-10-21(30-2)16-22(23)25(27)29)17-6-8-19(9-7-17)31-20-12-14-28(15-13-20)18-4-3-5-18/h6-11,16,18,20H,3-5,12-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246331

(2-(4-(1-cyclopentylpiperidin-4-yloxy)phenyl)-3-met...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C25H29N3O2/c1-27-24(26-23-9-5-4-8-22(23)25(27)29)18-10-12-20(13-11-18)30-21-14-16-28(17-15-21)19-6-2-3-7-19/h4-5,8-13,19,21H,2-3,6-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246290

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-3-meth...)Show SMILES Cn1c(nc2ccccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H27N3O2/c1-26-23(25-22-8-3-2-7-21(22)24(26)28)17-9-11-19(12-10-17)29-20-13-15-27(16-14-20)18-5-4-6-18/h2-3,7-12,18,20H,4-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246382

(8-chloro-2-(4-(1-cyclobutylpiperidin-4-yloxy)pheny...)Show SMILES Cn1c(nc2c(Cl)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26ClN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246240

(2-Methyl-3-(4-{[3-(1-piperidinyl)propyl]oxy}phenyl...)Show InChI InChI=1S/C23H27N3O2/c1-18-24-22-9-4-3-8-21(22)23(27)26(18)19-10-12-20(13-11-19)28-17-7-16-25-14-5-2-6-15-25/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50246287

(3-benzyl-2-(4-(3-(piperidin-1-yl)propoxy)phenyl)qu...)Show SMILES O=c1n(Cc2ccccc2)c(nc2ccccc12)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C29H31N3O2/c33-29-26-12-5-6-13-27(26)30-28(32(29)22-23-10-3-1-4-11-23)24-14-16-25(17-15-24)34-21-9-20-31-18-7-2-8-19-31/h1,3-6,10-17H,2,7-9,18-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human adrenergic alpha1A receptor expressed in LMtk- cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246240

(2-Methyl-3-(4-{[3-(1-piperidinyl)propyl]oxy}phenyl...)Show InChI InChI=1S/C23H27N3O2/c1-18-24-22-9-4-3-8-21(22)23(27)26(18)19-10-12-20(13-11-19)28-17-7-16-25-14-5-2-6-15-25/h3-4,8-13H,2,5-7,14-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50246434

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-fluo...)Show SMILES Cn1c(nc2c(F)cccc2c1=O)-c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C24H26FN3O2/c1-27-23(26-22-20(24(27)29)6-3-7-21(22)25)16-8-10-18(11-9-16)30-19-12-14-28(15-13-19)17-4-2-5-17/h3,6-11,17,19H,2,4-5,12-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50246435

(2-(4-(1-cyclobutylpiperidin-4-yloxy)phenyl)-8-meth...)Show SMILES COc1nccc2c1nc(-c1ccc(OC3CCN(CC3)C3CCC3)cc1)n(C)c2=O Show InChI InChI=1S/C24H28N4O3/c1-27-22(26-21-20(24(27)29)10-13-25-23(21)30-2)16-6-8-18(9-7-16)31-19-11-14-28(15-12-19)17-4-3-5-17/h6-10,13,17,19H,3-5,11-12,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H2 receptor |
Bioorg Med Chem Lett 18: 6041-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.034
BindingDB Entry DOI: 10.7270/Q2QJ7H5R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data